Textbook Question
Predict the chemical formula of the ionic compound formed between the following pairs of elements: (a) Al and Cl
1
views

 Verified step by step guidance
Verified step by step guidance
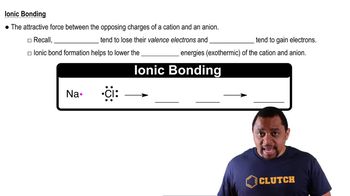
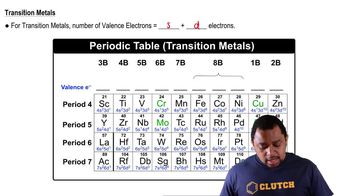

Predict the chemical formula of the ionic compound formed between the following pairs of elements: (a) Al and Cl
Predict the chemical formula of the ionic compound formed between the following pairs of elements: (b) Mg and O (c) Zn and Cl
Predict the chemical formula of the ionic compound formed between the following pairs of elements: (d) Li and O.
Which ionic compound is expected to form from combining the following pairs of elements? (d) aluminum and selenium.
Write the electron configuration for each of the following ions, and determine which ones possess noble-gas configurations: (a) Be2+, (b) Mn2+, (c) Cd2+, (d) Fe3+, (e) Tl+, (f) At-.
(a) Is lattice energy usually endothermic or exothermic?