NaCl and KF have the same crystal structure. The only difference between the two is the distance that separates cations and anions. (b) Use the ionic radii given in Figure 7.8 to estimate the Na─Cl and K─F distances.

The substances NaF and CaO are isoelectronic (have the same number of valence electrons). (c) Without looking up lattice energies, which compound is predicted to have the larger lattice energy?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
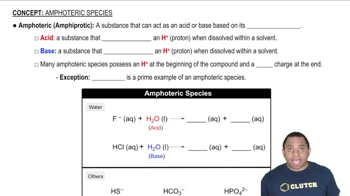
Key Concepts
Lattice Energy

Ionic Size and Charge

Isoelectronic Species
The substances NaF and CaO are isoelectronic (have the same number of valence electrons). (a) What are the charges on each of the cations in each compound?
The substances NaF and CaO are isoelectronic (have the same number of valence electrons). (b) What are the charges of each of the anions in each compound?
The substances NaF and CaO are isoelectronic (have the same number of valence electrons). (d) Using the lattice energies in Table 8.1, predict the lattice energy of ScN.
(a) Does the lattice energy of an ionic solid increase or decrease (i) as the charges of the ions increase, (ii) as the sizes of the ions increase?
(b) Arrange the following substances not listed in Table 8.1 according to their expected lattice energies, listing them from lowest lattice energy to the highest: MgS, KI, GaN, LiBr.
