Energy is required to remove two electrons from Ca to form Ca2+, and energy is required to add two electrons to O to form O2 - . Yet CaO is stable relative to the free elements. Which statement is the best explanation? (a) The lattice energy of CaO is large enough to overcome these processes. (b) CaO is a covalent compound, and these processes are irrelevant. (c) CaO has a higher molar mass than either Ca or O. (d) The enthalpy of formation of CaO is small. (e) CaO is stable to atmospheric conditions.
Ch.8 - Basic Concepts of Chemical Bonding

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 8, Problem 30b
(b) Using data from Appendix C, Figure 7.11, Figure 7.13, and the value of the second ionization energy for Ca, 1145 kJ/mol, calculate the lattice energy of CaCl2.
 Verified step by step guidance
Verified step by step guidance1
Identify the Born-Haber cycle for the formation of CaCl2, which involves the following steps: sublimation of Ca, ionization of Ca, dissociation of Cl2, and electron affinity of Cl.
Write the equation for the formation of CaCl2 from its elements in their standard states: Ca(s) + Cl2(g) -> CaCl2(s).
Use Hess's Law to relate the enthalpy changes of each step in the Born-Haber cycle to the lattice energy of CaCl2.
Calculate the enthalpy change for each step: sublimation of Ca, first and second ionization energies of Ca, bond dissociation energy of Cl2, and electron affinity of Cl.
Combine all the enthalpy changes using Hess's Law to solve for the lattice energy of CaCl2.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Lattice Energy
Lattice energy is the energy released when gaseous ions combine to form an ionic solid. It is a measure of the strength of the forces between the ions in an ionic compound. A higher lattice energy indicates a more stable ionic compound, as it reflects stronger ionic interactions. Lattice energy can be calculated using the Born-Haber cycle, which relates ionization energies, electron affinities, and the formation of the solid.
Recommended video:
Guided course

Lattice Energy
Ionization Energy
Ionization energy is the energy required to remove an electron from a gaseous atom or ion. The second ionization energy specifically refers to the energy needed to remove a second electron after the first has been removed. This value is crucial in determining the stability of the resulting ion and plays a significant role in calculating lattice energy, as it reflects the energy changes associated with ion formation.
Recommended video:
Guided course

Ionization Energy
Born-Haber Cycle
The Born-Haber cycle is a thermodynamic cycle that relates the lattice energy of an ionic compound to other measurable quantities, such as ionization energies, electron affinities, and enthalpy changes. It provides a systematic way to calculate lattice energy by considering the steps involved in forming an ionic solid from its constituent elements. This cycle is essential for understanding the energy changes that occur during the formation of ionic compounds like CaCl2.
Recommended video:
Guided course
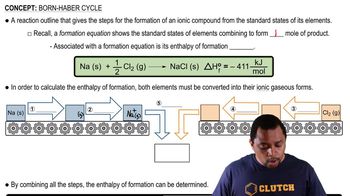
Born Haber Cycle
Related Practice
Textbook Question
1
views
Textbook Question
List the individual steps used in constructing a Born–Haber cycle for the formation of BaI2 from the elements. Which of the steps would you expect to be exothermic?
1
views
Textbook Question
(a) Based on the lattice energies of MgCl2 and SrCl2 given in Table 8.1, what is the range of values that you would expect for the lattice energy of CaCl2?
Textbook Question
(a) State whether or not the bonding in each substance is likely to be covalent: (i) glucose, (ii) nitrogen, (iii) aluminum hydroxide, (iv) ammonia, (v) neon.
Textbook Question
(b) A substance, XY, formed from two different elements, melts at −33 °C. Is XY likely to be a covalent or an ionic substance?
1
views
