The ionic compound CaO crystallizes with the same structure as sodium chloride (Figure 8.3). (a) In this structure, how many O2- are in contact with each Ca2+ ion (Hint: Remember the pattern of ions shown in Figure 8.3 repeats over and over again in all three directions.)
Ch.8 - Basic Concepts of Chemical Bonding

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 8, Problem 84
A classmate of yours is convinced that he knows everything about electronegativity. (a) In the case of atoms X and Y having different electronegativities, he says, the diatomic molecule X–Y must be polar. Is your classmate correct? (b) Your classmate says that the farther the two atoms are apart in a bond, the larger the dipole moment will be. Is your classmate correct?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of electronegativity. Electronegativity is the ability of an atom to attract shared electrons in a chemical bond. It varies across the periodic table, generally increasing from left to right and decreasing from top to bottom.
Step 2: Analyze part (a) of the problem. When two atoms, X and Y, have different electronegativities, the electrons in the bond are not shared equally, leading to a polar bond. However, the overall polarity of the molecule depends on its geometry. If the molecule is symmetrical, it might still be nonpolar despite having polar bonds.
Step 3: Consider part (b) of the problem. The dipole moment is a measure of the separation of positive and negative charges in a molecule. It depends on both the difference in electronegativity and the distance between the charges. However, the statement that the farther apart the atoms are, the larger the dipole moment, is not entirely correct. The dipole moment is also influenced by the magnitude of the charge difference.
Step 4: Clarify the relationship between bond length and dipole moment. While a longer bond length can contribute to a larger dipole moment, it is not the sole factor. The difference in electronegativity is crucial in determining the dipole moment.
Step 5: Summarize the findings. For part (a), a molecule with atoms of different electronegativities can be polar, but molecular geometry must be considered. For part (b), the dipole moment is influenced by both the electronegativity difference and the bond length, not just the distance between atoms.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electronegativity
Electronegativity is a measure of an atom's ability to attract and hold onto electrons in a chemical bond. It varies across the periodic table, generally increasing from left to right and decreasing from top to bottom. When two atoms with different electronegativities form a bond, the atom with the higher electronegativity will attract the shared electrons more strongly, leading to a polar bond.
Recommended video:
Guided course

Electronegativity Trends
Polarity of Molecules
A molecule is considered polar if it has a net dipole moment due to the uneven distribution of electron density. This occurs when there is a significant difference in electronegativity between the bonded atoms, resulting in partial positive and negative charges. Therefore, if atoms X and Y have different electronegativities, the diatomic molecule X–Y is indeed polar, confirming your classmate's assertion in part (a).
Recommended video:
Guided course

Molecular Polarity
Dipole Moment
The dipole moment is a quantitative measure of the polarity of a bond or molecule, defined as the product of the charge difference and the distance between the charges. It is represented as a vector pointing from the positive to the negative charge. Contrary to your classmate's claim in part (b), the dipole moment increases with greater electronegativity differences rather than distance alone; while distance does play a role, it is the charge separation that primarily influences the dipole moment.
Recommended video:
Guided course
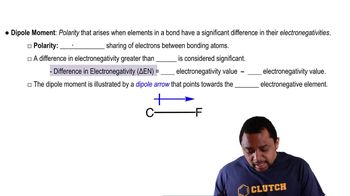
Dipole Moment
Related Practice
Textbook Question
Textbook Question
Construct a Born–Haber cycle for the formation of the hypothetical compound NaCl2, where the sodium ion has a 2+ charge (the second ionization energy for sodium is given in Table 7.2). (a) How large would the lattice energy need to be for the formation of NaCl2 to be exothermic?
Textbook Question
Consider the collection of nonmetallic elements O, P, Te, I, and B. (a) Which two would form the most polar single bond?
Textbook Question
Consider the collection of nonmetallic elements O, P, Te, I, and B. (b) Which two would form the longest single bond?
Textbook Question
Consider the collection of nonmetallic elements: B, As, O, and I. (d) Which element would likely to participate in two covalent bonds?
