Textbook Question
Estimate the As¬I bond length from the data in Figure 7.7 and compare your value to the experimental As ¬I bond length in arsenic triiodide, AsI3, 2.55 Å.

 Verified step by step guidance
Verified step by step guidance
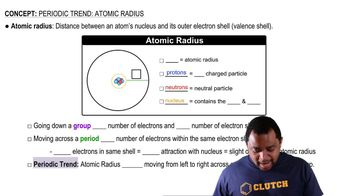

Estimate the As¬I bond length from the data in Figure 7.7 and compare your value to the experimental As ¬I bond length in arsenic triiodide, AsI3, 2.55 Å.
Using only the periodic table, arrange each set of atoms in order from largest to smallest: (a) Ar, As, Kr (b) Cd, Rb, Te (c) F, O, N.
Identify each statement as true or false: (a) Cations are larger than their corresponding neutral atoms.
Identify each statement as true or false: (b) Li+ is smaller than Li.
Identify each statement as true or false: (c) Cl- is bigger than I-.