Detailed calculations show that the value of Zeff for the outermost electrons in Na and K atoms is 2.51+ and 3.49+, respectively. (a) What value do you estimate for Zeff experienced by the outermost electron in both Na and K by assuming core electrons contribute 1.00 and valence electrons contribute 0.00 to the screening constant?

Detailed calculations show that the value of Zeff for the outermost electrons in Si and Cl atoms is 4.29+ and 6.12+, respectively. (b) What values do you estimate for Zeff using Slater’s rules? (c) Which approach gives a more accurate estimate of Zeff? (d) Which method of approximation more accurately accounts for the steady increase in Zeff that occurs upon moving left to right across a period? (e) Predict Zeff for a valence electron in P (phosphorus) based on the calculations for Si and Cl.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Effective Nuclear Charge (Zeff)
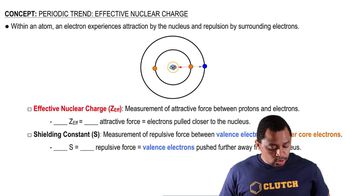
Slater's Rules
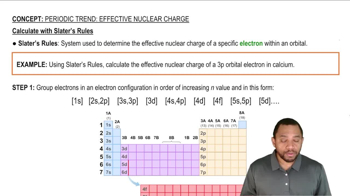
Trends in Zeff Across a Period

Detailed calculations show that the value of Zeff for the outermost electrons in Na and K atoms is 2.51+ and 3.49+, respectively. (b) What values do you estimate for Zeff using Slater’s rules?
Detailed calculations show that the value of Zeff for the outermost electrons in Na and K atoms is 2.51+ and 3.49+, respectively. (e) Predict Zeff for the outermost electrons in the Rb atom based on the calculations for Na and K.
Detailed calculations show that the value of Zeff for the outermost electrons in Si and Cl atoms is 4.29+ and 6.12+, respectively. (a) What value do you estimate for Zeff experienced by the outermost electron in both Si and Cl by assuming core electrons contribute 1.00 and valence electrons contribute 0.00 to the screening constant?
