Write balanced equations for the following reactions: (a) boron trichloride with water (b) cobalt (II) oxide with nitric acid (d) carbon dioxide with aqueous barium hydroxide.
Ch.7 - Periodic Properties of the Elements

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 7, Problem 68
Copper and calcium both form +2 ions, but copper is far less reactive. Suggest an explanation, taking into account the ground-state electron configurations of these elements and their atomic radii.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the ground-state electron configurations of copper (Cu) and calcium (Ca). Copper has the electron configuration [Ar] 3d^10 4s^1, while calcium has [Ar] 4s^2.
Step 2: Consider the electron removal process for both elements to form +2 ions. For copper, electrons are removed from the 4s and then the 3d orbitals, while for calcium, electrons are removed from the 4s orbital.
Step 3: Analyze the stability of the electron configurations after ionization. Copper achieves a stable, filled 3d^10 configuration after losing two electrons, which contributes to its lower reactivity.
Step 4: Compare the atomic radii of copper and calcium. Copper has a smaller atomic radius than calcium, which means its valence electrons are held more tightly by the nucleus, reducing its reactivity.
Step 5: Conclude that the filled d-subshell in copper and its smaller atomic radius contribute to its lower reactivity compared to calcium, which has a larger atomic radius and lacks a filled d-subshell.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electron Configuration
The electron configuration of an element describes the distribution of electrons in its atomic orbitals. For copper (Cu), the ground-state configuration is [Ar] 3d10 4s1, while calcium (Ca) has a configuration of [Ar] 4s2. The presence of a filled d-subshell in copper contributes to its stability and lower reactivity compared to calcium, which has two electrons in its outermost s-subshell that are more easily lost.
Recommended video:
Guided course

Electron Configuration Example
Atomic Radius
Atomic radius refers to the size of an atom, typically measured from the nucleus to the outermost electron shell. Copper has a smaller atomic radius than calcium due to its higher nuclear charge, which pulls the electrons closer to the nucleus. A smaller atomic radius can lead to stronger attraction between the nucleus and the valence electrons, making it more difficult for copper to lose electrons and thus reducing its reactivity.
Recommended video:
Guided course
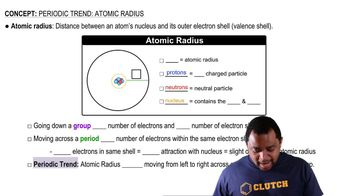
Atomic Radius
Reactivity of Metals
The reactivity of metals is influenced by their ability to lose electrons and form positive ions. Metals with fewer valence electrons, like calcium, tend to be more reactive because they can easily lose these electrons to achieve a stable electron configuration. In contrast, copper's filled d-subshell and smaller atomic radius make it less inclined to lose its single 4s electron, resulting in lower reactivity compared to calcium.
Recommended video:
Guided course

Transition Metals
Related Practice
Textbook Question
Textbook Question
Write balanced equations for the following reactions: (a) sulfur dioxide with water (b) lithium oxide in water (c) zinc oxide with dilute hydrochloric acid (d) arsenic trioxide with aqueous potassium hydroxide.
Textbook Question
Write a balanced equation for the reaction that occurs in each of the following cases: (b) Strontium oxide is added to water. (c) A fresh surface of lithium metal is exposed to oxygen gas. (d) Sodium metal reacts with molten sulfur.
1
views
Textbook Question
Write a balanced equation for the reaction that occurs in each of the following cases: (a) Potassium metal is exposed to an atmosphere of chlorine gas.
1
views
Textbook Question
Write a balanced equation for the reaction that occurs in each of the following cases: (a) Cesium is added to water.
1
views
