Textbook Question
Explain the variation in the ionization energies of carbon, as displayed in this graph:

 Verified step by step guidance
Verified step by step guidance

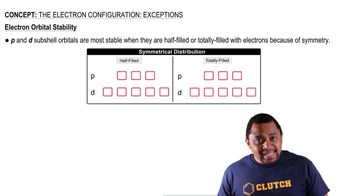
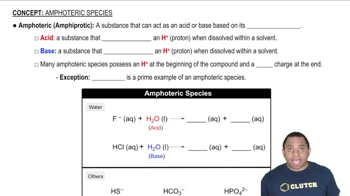
Explain the variation in the ionization energies of carbon, as displayed in this graph:
In the chemical process called electron transfer, an electron is transferred from one atom or molecule to another. (We will talk about electron transfer extensively in Chapter 20.) A simple electron transfer reaction is A(g) + A(g) → A+(g) + A-(g) For a representative nonmetal such as chlorine, is this process exothermic?
(a) Use orbital diagrams to illustrate what happens when an oxygen atom gains two electrons
Identify two ions that have the following ground-state electron configurations: (a) [Ar]
Identify two ions that have the following ground-state electron configurations: (b) [Ar]3d5