Little is known about the properties of astatine, At, because of its rarity and high radioactivity. Nevertheless, it is possible for us to make many predictions about its properties. (b) Would you expect At to be a metal, nonmetal, or metalloid? Explain.
Ch.7 - Periodic Properties of the Elements

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 7, Problem 76
(b) Using appropriate reference sources, look up the bond lengths of Xe—F bonds in several molecules. How do these numbers compare to the bond lengths calculated from the atomic radii of the elements?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the molecules containing Xe—F bonds. Common examples include xenon difluoride (XeF2), xenon tetrafluoride (XeF4), and xenon hexafluoride (XeF6).
Step 2: Use a reliable reference source, such as a chemistry database or textbook, to find the experimental bond lengths of Xe—F in these molecules. Note that bond lengths can vary slightly depending on the molecular environment.
Step 3: Calculate the theoretical bond length using the atomic radii of xenon and fluorine. The bond length can be estimated as the sum of the covalent radii of the two atoms: Bond length = r(Xe) + r(F).
Step 4: Compare the experimental bond lengths obtained from the reference sources with the calculated bond lengths from atomic radii. Discuss any discrepancies and consider factors such as molecular geometry and electron cloud interactions that might affect the bond length.
Step 5: Reflect on the limitations of using atomic radii to predict bond lengths, considering that atomic radii are average values and do not account for specific molecular interactions.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Bond Length
Bond length is the average distance between the nuclei of two bonded atoms. It is influenced by the size of the atoms involved and the type of bond (single, double, or triple). In the case of Xe—F bonds, bond lengths can vary depending on the molecular environment and hybridization of the atoms.
Recommended video:
Guided course

Average Bond Order
Atomic Radii
Atomic radii refer to the size of an atom, typically measured from the nucleus to the outermost electron shell. These values can vary based on the atom's state (e.g., covalent, ionic) and its position in the periodic table. Understanding atomic radii is essential for predicting bond lengths, as they provide a baseline for the expected distance between bonded atoms.
Recommended video:
Guided course
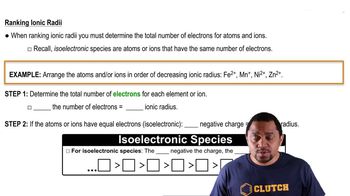
Ranking Ionic Radii
Comparison of Experimental and Calculated Values
Comparing experimental bond lengths with those calculated from atomic radii helps assess the accuracy of theoretical models in chemistry. Discrepancies may arise due to factors like electron repulsion, molecular geometry, and the presence of lone pairs, which can affect the actual bond length in a molecule compared to simple calculations based on atomic sizes.
Recommended video:
Guided course
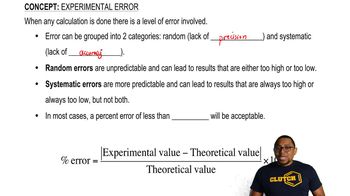
Experimental Error
Related Practice
Textbook Question
Textbook Question
Little is known about the properties of astatine, At, because of its rarity and high radioactivity. Nevertheless, it is possible for us to make many predictions about its properties. (c) What is the chemical formula of the compound it forms with Na?
Textbook Question
Write a balanced equation for the reaction that occurs in each of the following cases: (a) White phorphrous, P4(s), reacts with chlorine gas. (b) Sodium metal reacts with water. (c) Sulfur reacts with hydrogen gas.
1
views
Textbook Question
Write a balanced equation for the reaction that occurs in each of the following cases: (d) Fluorine reacts with water.
1
views
