The prefix eka- comes from the Sanskrit word for 'one.' Mendeleev used this prefix to indicate that the unknown element was one place away from the known element that followed the prefix. For example, eka-silicon, which we now call germanium, is one element below silicon. Mendeleev also predicted the existence of eka-manganese, which was not experimentally confirmed until 1937 because this element is radioactive and does not occur in nature. Based on the periodic table shown in Figure 7.1, what do we now call the element Mendeleev called eka-manganese?

Among the elements N, O, P, and S, which element or elementshave the smallest effect nuclear charge if we use Equation7.1 to calculate Zeff? Which element or elements havethe largest effective nuclear charge?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Effective Nuclear Charge (Zeff)
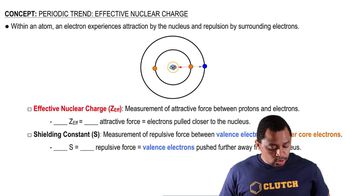
Shielding Effect
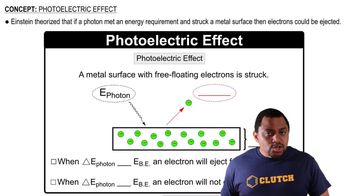
Trends in the Periodic Table
Which of the following statements about effective nuclear charge for the outermost valence electron of an atom is incorrect? (i) The effective nuclear charge can be thought of as the true nuclear charge minus a screening constant due to the other electrons in the atom. (ii) Effective nuclear charge increases going left to right across a row of the periodic table. (iii) Valence electrons screen the nuclear charge more effectively than do core electrons. (iv) The effective nuclear charge shows a sudden decrease when we go from the end of one row to the beginning of the next row of the periodic table. (v) The change in effective nuclear charge going down a column of the periodic table is generally less than that going across a row of the periodic table
Detailed calculations show that the value of Zeff for the outermost electrons in Na and K atoms is 2.51+ and 3.49+, respectively. (a) What value do you estimate for Zeff experienced by the outermost electron in both Na and K by assuming core electrons contribute 1.00 and valence electrons contribute 0.00 to the screening constant?
Detailed calculations show that the value of Zeff for the outermost electrons in Na and K atoms is 2.51+ and 3.49+, respectively. (b) What values do you estimate for Zeff using Slater’s rules?
