Molybdenum metal must absorb radiation with an energy higher than 7.22 * 10-19 J ('energy threshold') before it can eject an electron from its surface via the photoelectric effect. (a) What is the frequency threshold for emission of electrons?

Titanium metal requires light with a maximum wavelength of 286 nm to emit electrons. (a) What is the minimum energy of the photons necessary to emit electrons from titanium via the photoelectric effect? (b) If titanium is irradiated with light of wavelength 276 nm, what is the maximum possible kinetic energy of the emitted electrons?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Photoelectric Effect
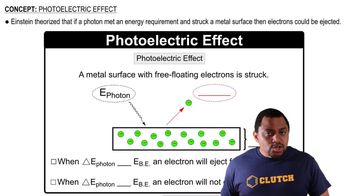
Photon Energy Calculation

Kinetic Energy of Emitted Electrons
Molybdenum metal must absorb radiation with an energy higher than 7.22 * 10-19 J ('energy threshold') before it can eject an electron from its surface via the photoelectric effect. (b) What wavelength of radiation will provide a photon of this energy?
Molybdenum metal must absorb radiation with an energy higher than 7.22 * 10-19 J ('energy threshold') before it can eject an electron from its surface via the photoelectric effect. (c) If molybdenum is irradiated with light of wavelength of 240 nm, what is the maximum possible velocity of the emitted electrons?
Does the hydrogen atom 'expand' or 'contract' when an electron is excited from the n = 1 state to the n = 3 state?
Classify each of the following statements as either true or false: (a) A hydrogen atom in the n = 3 state can emit light at only two specific wavelengths (b) a hydrogen atom in the n = 2 state is at a lower energy than one in the n = 1 state (c) the energy of an emitted photon equals the energy difference of the two states involved in the emission.
Is energy emitted or absorbed when the following electronic transitions occur in hydrogen? (a) from n = 3 to n = 2 (c) an electron adds to the H+ ion and ends up in the n = 2 shell?
