For the table that follows, write which orbital goes with the quantum numbers. Don't worry about x, y, z subscripts. If the quantum numbers are not allowed, write 'not allowed.' n l ml Orbital 2 1 -1 2p (example) 1 0 0 3 -3 2 3 2 -2 2 0 -1 0 0 0 4 2 1 5 3 0
Ch.6 - Electronic Structure of Atoms

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 6, Problem 65
(b) In what sense does a 2p orbital have directional character? Compare the 'directional' characteristics of the px and dx² - y² orbitals. (That is, in what direction or region of space is the electron density concentrated?)
 Verified step by step guidance
Verified step by step guidance1
Understand the concept of atomic orbitals: Atomic orbitals are regions in space where there is a high probability of finding an electron. Each type of orbital (s, p, d, f) has a unique shape and orientation.
Describe the 2p orbital: The 2p orbitals are dumbbell-shaped and have directional character because they are oriented along the x, y, or z axes. This means that the electron density is concentrated along these axes, giving them a specific direction in space.
Explain the px orbital: The px orbital is one of the three 2p orbitals, and it is oriented along the x-axis. The electron density is concentrated in two lobes on either side of the nucleus along the x-axis, indicating its directional character.
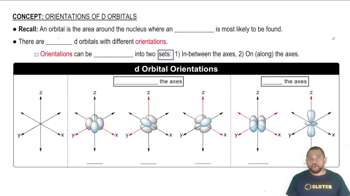
Describe the dx² - y² orbital: The dx² - y² orbital is one of the five d orbitals. It has a cloverleaf shape with lobes lying along the x and y axes. The electron density is concentrated in these lobes, making it directional, but in a different way compared to the p orbitals.
Compare the directional characteristics: While the px orbital has electron density concentrated along the x-axis, the dx² - y² orbital has electron density concentrated along both the x and y axes. This means the dx² - y² orbital has a more complex directional character, with electron density in regions between the axes as well.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Orbital Shapes and Orientation
Orbitals are regions in an atom where there is a high probability of finding electrons. The 2p orbitals, which include px, py, and pz, have distinct shapes and orientations in three-dimensional space. The px orbital, for example, is oriented along the x-axis, while the py and pz orbitals are oriented along the y and z axes, respectively. This directional character influences how atoms interact and bond with one another.
Recommended video:
Guided course
d Orbital Orientations
Electron Density Distribution
Electron density refers to the probability of finding an electron in a particular region of space around the nucleus. In the case of the px orbital, the electron density is concentrated along the x-axis, while the dx² - y² orbital has a unique shape that focuses electron density along the x and y axes, forming lobes in the quadrants of the xy-plane. Understanding these distributions is crucial for predicting chemical bonding and molecular geometry.
Recommended video:
Guided course
Velocity Distributions
Comparison of Orbital Characteristics
When comparing the px and dx² - y² orbitals, it is important to note their different shapes and electron density distributions. The px orbital has a simple lobular shape along the x-axis, while the dx² - y² orbital has a more complex shape with four lobes oriented towards the corners of the xy-plane. This difference in orientation and shape affects how these orbitals overlap with others during chemical bonding, influencing molecular structure and reactivity.
Recommended video:
Guided course
Molecular Orbital Theory
Related Practice
Textbook Question
1
views
Textbook Question
Sketch the shape and orientation of the following types of orbitals: (a) s, (b) pz, (c) dxy.
Textbook Question
Sketch the shape and orientation of the following types of orbitals: (a) px, (b) dz2, (c) dx2 - y2.
1
views
Textbook Question
(c) What can you say about the average distance from the nucleus of an electron in a 2s orbital as compared with a 3s orbital?
Textbook Question
(d) For the hydrogen atom, list the following orbitals in order of increasing energy (that is, most stable ones first): 4f, 6s, 3d, 1s, 2p.
Textbook Question
(a) With reference to Figure 6.19, what is the relationship between the number of nodes in an s orbital and the value of the principal quantum number?
