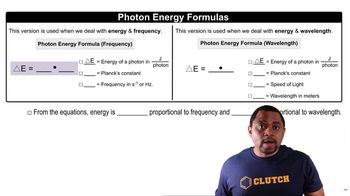
Textbook Question
If human height were quantized in 1-cm increments, what would happen to the height of a child as she grows up: (i) the child's height would never change, (ii) the child's height would continuously increase, (iii) the child's height would increase in jumps of 6 cm, or (iv) the child's height would increase in 'jumps' of 1 cm at a time?
1
views