Bohr's model can be used for hydrogen-like ions—ions that have only one electron, such as He+ and Li2+. (a) Why is the Bohr model applicable to He+ ions but not to neutral He atoms?
Ch.6 - Electronic Structure of Atoms

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 6, Problem 97
As discussed in the A Closer Look box on “Measurement and the Uncertainty Principle,” the essence of the uncertainty principle is that we can’t make a measurement without disturbing the system that we are measuring. (b) How is this concept related to the paradox discussed in the Closer Look box on “Thought Experiments and Schrödinger’s Cat”?
 Verified step by step guidance
Verified step by step guidance1
The uncertainty principle, formulated by Werner Heisenberg, states that it is impossible to simultaneously know both the exact position and exact momentum of a particle. This principle highlights the fundamental limits of precision in measurement at the quantum level.
Schrödinger's Cat is a thought experiment that illustrates the concept of superposition in quantum mechanics. It describes a scenario where a cat is placed in a sealed box with a radioactive atom, a Geiger counter, and a vial of poison. The cat's fate is linked to the decay of the atom, which is a random event.
In the context of Schrödinger's Cat, the cat is considered to be in a superposition of states (both alive and dead) until an observation is made. This ties into the uncertainty principle, as the act of measurement (opening the box) disturbs the system and forces it into one of the possible states.
The paradox arises because, according to classical intuition, the cat should be either alive or dead, not both. However, quantum mechanics suggests that until a measurement is made, the system exists in all possible states simultaneously.
Thus, the uncertainty principle and Schrödinger's Cat both emphasize the role of the observer in determining the state of a quantum system, highlighting the intrinsic limitations and peculiarities of measuring quantum phenomena.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Uncertainty Principle
The uncertainty principle, formulated by Werner Heisenberg, states that certain pairs of physical properties, like position and momentum, cannot be simultaneously measured with arbitrary precision. This inherent limitation arises because the act of measuring one property disturbs the other, leading to a fundamental limit on our ability to predict the behavior of quantum systems.
Recommended video:
Guided course
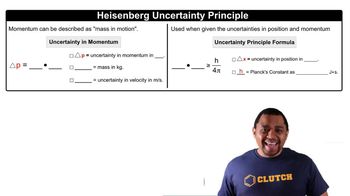
Uncertainty Principle Formula
Schrödinger's Cat
Schrödinger's Cat is a thought experiment that illustrates the concept of superposition in quantum mechanics. It describes a scenario where a cat in a sealed box can be simultaneously alive and dead until an observation is made. This paradox highlights the strange implications of quantum measurement and the role of the observer in determining the state of a system.
Recommended video:
Guided course

Constant-Pressure Calorimetry Example 2
Measurement Disturbance
Measurement disturbance refers to the phenomenon where the act of measuring a quantum system alters its state. This is crucial in understanding both the uncertainty principle and Schrödinger's Cat, as it emphasizes that observations in quantum mechanics are not passive; they actively influence the system being observed, leading to outcomes that challenge classical intuitions about reality.
Recommended video:
Guided course
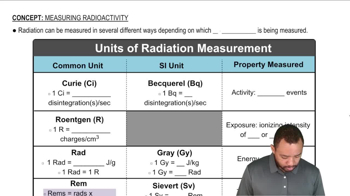
Units of Radiation Measurement
Related Practice
Textbook Question
Textbook Question
As discussed in the A Closer Look box on 'Measurement and the Uncertainty Principle,' the essence of the uncertainty principle is that we can't make a measurement without disturbing the system that we are measuring. (a) Why can't we measure the position of a subatomic particle without disturbing it?
1
views
Textbook Question
Consider the discussion of radial probability functions in'A Closer Look' in Section 6.6. (a) What is the differencebetween the probability density as a function of r and theradial probability function as a function of r ?
1
views
Textbook Question
The Chemistry and Life box in Section 6.7 described the techniques called NMR and MRI. (a) Instruments for obtaining MRI data are typically labeled with a frequency, such as 600 MHz. In what region of the electromagnetic spectrum does a photon with this frequency belong?
