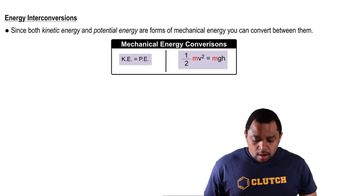
Textbook Question
The accompanying photo shows a pipevine swallowtail caterpillar climbing up a twig. (a) As the caterpillar climbs, its potential energy is increasing. What source of energy has been used to effect this change in potential energy?
4
views