(d) How many kJ of heat are needed to raise the temperature of 5.00 kg of liquid water from 24.6 to 46.2 °C?
Ch.5 - Thermochemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 5, Problem 53b
The specific heat of octane, C8H18(l), is 2.22 J•g/K. (b) Which will require more heat, increasing the temperature of 1 mol of C8H18(l), by a certain amount or increasing the temperature of 1 mol of H2O(l) by the same amount?
 Verified step by step guidance
Verified step by step guidance1
First, determine the molar mass of octane, C8H18. The molar mass is calculated by adding the atomic masses of all the atoms in the molecule: 8 carbons (C) and 18 hydrogens (H).
Calculate the total heat required to increase the temperature of 1 mol of octane by a certain temperature change, \(\Delta T\). Use the formula: \(q = m \cdot c \cdot \Delta T\), where \(m\) is the mass of the substance, \(c\) is the specific heat, and \(\Delta T\) is the temperature change.
Next, find the specific heat of water, which is typically 4.18 J/g/K. Calculate the total heat required to increase the temperature of 1 mol of water by the same temperature change, \(\Delta T\), using the same heat formula.
Compare the total heat required for octane and water. The substance requiring more heat to achieve the same temperature change will have a higher product of its mass and specific heat.
Conclude which substance, octane or water, requires more heat to increase the temperature by the same amount based on the calculations from the previous steps.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Specific Heat Capacity
Specific heat capacity is the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius (or Kelvin). It is a crucial property that varies between different substances, influencing how much energy is needed to change their temperature. In this question, the specific heat of octane is given, which allows for comparison with water's specific heat to determine which substance requires more heat for the same temperature change.
Recommended video:
Guided course
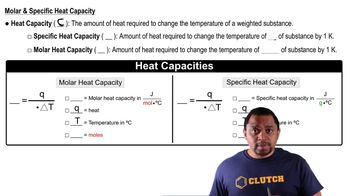
Heat Capacity
Molar Mass and Moles
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). Understanding moles is essential in chemistry as it allows for the conversion between mass and the number of particles. In this question, both octane and water are considered in terms of moles, which helps in calculating the total heat required for temperature changes based on their specific heat capacities.
Recommended video:
Guided course

Molar Mass Concept
Heat Transfer and Energy Calculations
Heat transfer refers to the movement of thermal energy from one object or substance to another, often quantified using the formula Q = mcΔT, where Q is the heat added, m is the mass, c is the specific heat capacity, and ΔT is the change in temperature. This concept is fundamental in determining how much heat is needed to raise the temperature of a substance, allowing for a direct comparison between octane and water in the context of the question.
Recommended video:
Guided course
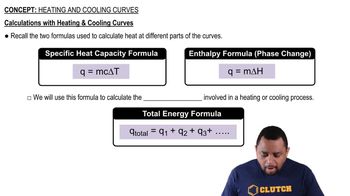
Calculations with Heating and Cooling Curves
Related Practice
Textbook Question
Textbook Question
(b) Calculate the energy needed for this temperature change.
Textbook Question
The specific heat of octane, C8H18(l), is 2.22 J•g/K. (a) How many J of heat are needed to raise the temperature of 80.0 g of octane from 10.0 to 25.0 °C?
Textbook Question
Consider the data about gold metal in Exercise 5.26(b). (a) Based on the data, calculate the specific heat of Au(s).
Textbook Question
Consider the data about gold metal in Exercise 5.26(b). (b) Suppose that the same amount of heat is added to two 10.0-g blocks of metal, both initially at the same temperature. One block is gold metal, and one is iron metal. Which block will have the greater rise in temperature after the addition of the heat?
Textbook Question
Consider the data about gold metal in Exercise 5.26(b). (c) What is the molar heat capacity of Au(s)?
