Imagine that you are climbing a mountain. (b) Is the change in elevation between your base camp and the peak a state function?
Ch.5 - Thermochemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 5, Problem 6c
The diagram shows four states of a system, each with different internal energy, E. (c) Write an expression for the difference in energy between State C and State D.
 Verified step by step guidance
Verified step by step guidance1
Identify the internal energies of State C and State D, denoted as E_C and E_D respectively.
Understand that the difference in energy between two states is calculated by subtracting the internal energy of one state from the other.
Write the expression for the difference in energy between State C and State D as \( \Delta E = E_C - E_D \).
Ensure that the signs are correct: if E_C is greater than E_D, \( \Delta E \) will be positive, indicating energy is released when moving from C to D.
If E_C is less than E_D, \( \Delta E \) will be negative, indicating energy is absorbed when moving from C to D.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Internal Energy
Internal energy is the total energy contained within a system, encompassing both kinetic and potential energy of the particles. It is a state function, meaning it depends only on the current state of the system, not on how it reached that state. Understanding internal energy is crucial for analyzing thermodynamic processes and energy changes in a system.
Recommended video:
Guided course

Internal Energy
State Function
A state function is a property of a system that depends only on its current state, not on the path taken to reach that state. Examples include internal energy, enthalpy, and pressure. In the context of the question, the difference in internal energy between two states (C and D) can be calculated directly from their respective energy values, illustrating the concept of state functions.
Recommended video:
Guided course
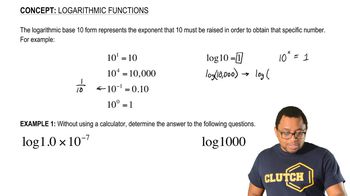
Logarithmic Functions
Energy Difference Calculation
Calculating the energy difference between two states involves subtracting the internal energy of one state from that of another. In this case, the expression for the difference in energy between State C and State D can be represented as ΔE = E_C - E_D. This calculation is fundamental in thermodynamics, allowing for the analysis of energy transfers and transformations within a system.
Recommended video:
Guided course

Gibbs Free Energy of Reactions
Related Practice
Textbook Question
5
views
Textbook Question
The diagram shows four states of a system, each with different internal energy, E. (a) Which of the states of the system has the greatest internal energy?
Textbook Question
Imagine a container placed in a tub of water, as depicted in the accompanying diagram. (a) If the contents of the container are the system and heat is able to flow through the container walls, what qualitative changes will occur in the temperatures of the system and in its surroundings? From the system's perspective, is the process exothermic or endothermic?
1
views
Textbook Question
In the accompanying cylinder diagram, a chemical process occurs at constant temperature and pressure. (a) Is the sign of w indicated by this change positive or negative?
2
views
