Use bond enthalpies in Table 5.4 to estimate H for each of the following reactions: (a)
Ch.5 - Thermochemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 5, Problem 87
Consider the reaction 2 H2(g) + O2(g) → 2 H2O(l). (b) Without doing a calculation, predict whether your estimate in part (a) is more negative or less negative than the true reaction enthalpy.
 Verified step by step guidance
Verified step by step guidance1
Understand that the reaction given is the formation of water from hydrogen and oxygen gases. This is an exothermic reaction, meaning it releases heat.
Recall that the enthalpy change for a reaction (
) is negative for exothermic reactions because energy is released.
Consider the bond energies involved: breaking bonds in reactants (H-H and O=O) requires energy, while forming bonds in products (O-H in water) releases energy.
Recognize that the bond formation in water releases more energy than the energy required to break the bonds in hydrogen and oxygen, leading to a net release of energy.
Conclude that if your estimate in part (a) was based on average bond energies, it might be less negative than the true reaction enthalpy because specific bond energies in the actual reaction could lead to a greater release of energy.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Enthalpy of Reaction
The enthalpy of reaction, or reaction enthalpy, is the heat change associated with a chemical reaction at constant pressure. It indicates whether a reaction is exothermic (releases heat, negative enthalpy change) or endothermic (absorbs heat, positive enthalpy change). Understanding this concept is crucial for predicting the energy changes that occur during the formation of products from reactants.
Recommended video:
Guided course

Enthalpy of Formation
Hess's Law
Hess's Law states that the total enthalpy change for a reaction is the same, regardless of the number of steps taken to achieve the reaction. This principle allows chemists to calculate the enthalpy change of a reaction by summing the enthalpy changes of individual steps, making it essential for estimating reaction enthalpies without direct measurements.
Recommended video:
Guided course
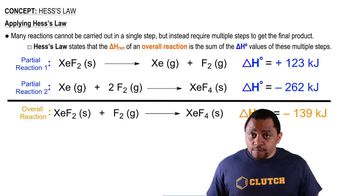
Hess's Law
Bond Enthalpies
Bond enthalpy refers to the amount of energy required to break a bond between two atoms in a molecule. It is a key factor in determining the overall enthalpy change of a reaction, as breaking bonds requires energy (endothermic) and forming bonds releases energy (exothermic). Understanding bond enthalpies helps in estimating whether the overall reaction enthalpy will be more or less negative than an initial estimate.
Recommended video:
Guided course

Enthalpy of Formation
Related Practice
Textbook Question
Textbook Question
(a) The nitrogen atoms in an N2 molecule are held together by a triple bond; use enthalpies of formation in Appendix C to estimate the enthalpy of this bond, D(N‚N). (b) Consider the reaction between hydrazine and hydrogen to produce ammonia, N2H41g2 + H21g2¡2 NH31g2. Use enthalpies of formation and bond enthalpies to estimate the enthalpy of the nitrogen– nitrogen bond in N2H4. (c) Based on your answers to parts (a) and (b), would you predict that the nitrogen–nitrogen bond in hydrazine is weaker than, similar to, or stronger than the bond in N2 ?
Textbook Question
Consider the reaction 2 H2(g) + O2(g) → 2 H2O(l). (a) Use the bond enthalpies in Table 5.4 to estimate H for this reaction, ignoring the fact that water is in the liquid state.
