Consider a system consisting of the following apparatus, in which gas is confined in one flask and there is a vacuum in the other flask. The flasks are separated by a valve. Assume that the flasks are perfectly insulated and will not allow the flow of heat into or out of the flasks to the surroundings. When the valve is opened, gas flows from the filled flask to the evacuated one. (a) Is work performed during the expansion of the gas? (b) Why or why not?

Both oxyhydrogen torches and fuel cells use the following reaction to produce energy: 2 H2(g) + O2(g) → 2 H2O(l). Both processes occur at constant pressure. In both cases, the change in state of the system is the same: the reactant is oxyhydrogen (“Knallgas”) and the product is water. Yet, with an oxyhydrogen torch, the heat evolved is large, and with a fuel cell, it is small. If heat at constant pressure is considered to be a state function, why does it depend on path?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
State Functions vs. Path Functions
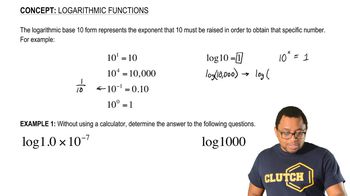
Enthalpy Change (ΔH)

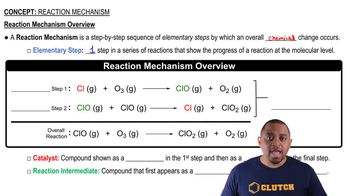
Reaction Mechanism and Energy Transfer
A sample of gas is contained in a cylinder-and-piston arrangement. There is an external pressure of 100 kPa. The gas undergoes the change in state shown in the drawing. (b) Now assume that the cylinder and piston are made up of a thermal conductor such as a metal. During the state change, the cylinder gets colder to the touch. What is the sign of q for the state change in this case? Describe the difference in the state of the system at the end of the process in the two cases. What can you say about the relative values of E?
A house is designed to have passive solar energy features. Brickwork incorporated into the interior of the house acts as a heat absorber. Each brick weighs approximately 1.8 kg. The specific heat of the brick is 0.85 J/g•K. How many bricks must be incorporated into the interior of the house to provide the same total heat capacity as 1.7⨉103 gal of water?
A coffee-cup calorimeter of the type shown in Figure 5.18 contains 150.0 g of water at 25.1°C A 121.0-g block of copper metal is heated to 100.4°C by putting it in a beaker of boiling water. The specific heat of Cu(s) is 0.385 J/g-K The Cu is added to the calorimeter, and after a time the contents of the cup reach a constant temperature of 30.1°C. (a) Determine the amount of heat, in J, lost by the copper block.
A coffee-cup calorimeter of the type shown in Figure 5.18 contains 150.0 g of water at 25.1°C A 121.0-g block of copper metal is heated to 100.4°C by putting it in a beaker of boiling water. The specific heat of Cu(s) is 0.385 J/g-K The Cu is added to the calorimeter, and after a time the contents of the cup reach a constant temperature of 30.1°C (b) Determine the amount of heat gained by the water. The specific heat of water is 4.184 J/1gK.
