For each of the following compounds, write a balanced thermochemical equation depicting the formation of one mole of the compound from its elements in their standard states and then look up ΔH°f for each substance in Appendix C. (a) NO2(g) (b) SO3(g) (c) NaBr(s) (d) Pb(NO3)2(s).

Acetylene (C2H2(g)) is used for welding because oxyacetylene is the hottest burning common fuel gas. Using standard enthalpies of formation, calculate the quantity of heat produced when 10 g of acetylene is completely combusted in air under standard conditions.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
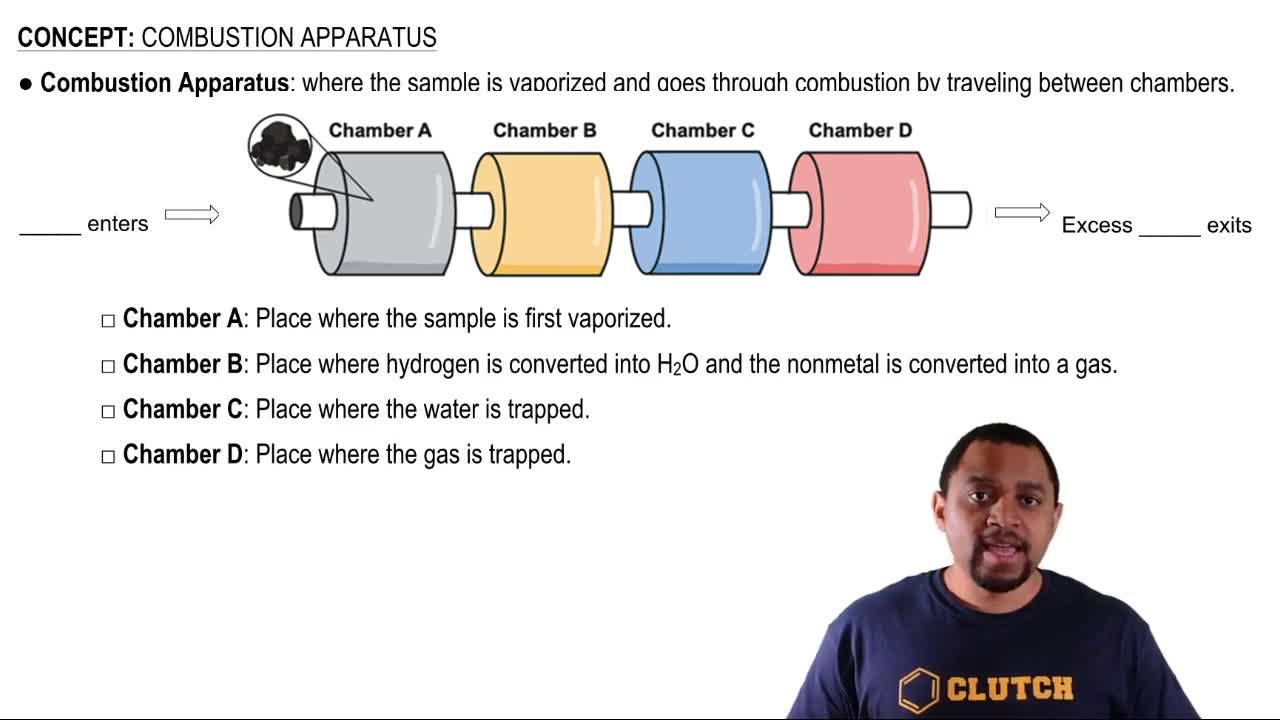
Key Concepts
Standard Enthalpy of Formation

Combustion Reaction
Heat of Combustion

Write balanced equations that describe the formation of the following compounds from elements in their standard states, and then look up the standard enthalpy of formation for each substance in Appendix C: (a) CH3OH(l)
Using values from Appendix C, calculate the value of H for each of the following reactions: (a) CaO(s) + 2 HF(g) → CaF2(s) + H2O(g) (b) Fe2O3(s) + 3 C(s) → 2 Fe(s) + 3CO(g) (c) 2 CO(g) + 2 NO(g) → N2(s) + 2 CO2(g) (d) 4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2Og)
Complete combustion of 1 mol of acetone (C3H6O) liberates 1790 kJ: C3H6O(l) + 4 O2(g) → 3 CO2(g) + 3 H2O(l) ΔH° = -1790 kJ Using this information together with the standard enthalpies of formation of O2(g), CO2(g), and H2O(l) from Appendix C, calculate the standard enthalpy of formation of acetone.
