Textbook Question
(b) Why does increasing the temperature cause a solid substance to change in succession from a solid to a liquid to a gas?
2
views

 Verified step by step guidance
Verified step by step guidance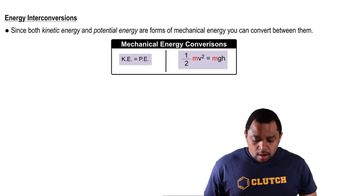

(b) Why does increasing the temperature cause a solid substance to change in succession from a solid to a liquid to a gas?
Consider the two diagrams that follow. (d) Would similar relationships hold for the work involved in each process?
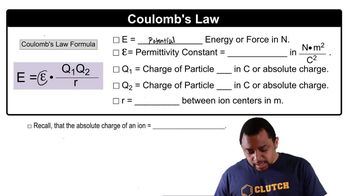
(a) What is the electrostatic potential energy (in joules) between two electrons that are separated by 62 pm?
(b) What is the change in potential energy if the distance separating the two electrons is increased to 1.0 nm?
(c) Does the potential energy of the two particles increase or decrease when the distance is increased to 1.0 nm?