
(a) Use enthalpies of formation given in Appendix C to calculate _x001F_H for the reaction Br2(g) → 2 Br(g), and use this value to estimate the bond enthalpy D(Br–Br). (b) How large is the difference between the value calculated in part (a) and the value given in Table 5.4?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Enthalpy of Formation

Bond Enthalpy

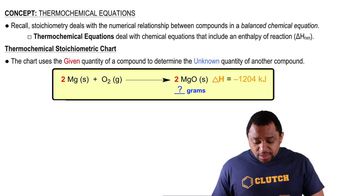
Thermochemical Equations
Use bond enthalpies in Table 5.4 to estimate H for each of the following reactions: (a)
(a) The nitrogen atoms in an N2 molecule are held together by a triple bond; use enthalpies of formation in Appendix C to estimate the enthalpy of this bond, D(N‚N). (b) Consider the reaction between hydrazine and hydrogen to produce ammonia, N2H41g2 + H21g2¡2 NH31g2. Use enthalpies of formation and bond enthalpies to estimate the enthalpy of the nitrogen– nitrogen bond in N2H4. (c) Based on your answers to parts (a) and (b), would you predict that the nitrogen–nitrogen bond in hydrazine is weaker than, similar to, or stronger than the bond in N2 ?
Consider the reaction 2 H2(g) + O2(g) → 2 H2O(l). (a) Use the bond enthalpies in Table 5.4 to estimate H for this reaction, ignoring the fact that water is in the liquid state.
