The arsenic in a 1.22-g sample of a pesticide was converted to AsO43- by suitable chemical treatment. It was then titrated using Ag+ to form Ag3AsO4 as a precipitate. (a) What is the oxidation state of As in AsO43-?

The arsenic in a 1.22-g sample of a pesticide was converted to AsO43- by suitable chemical treatment. It was then titrated using Ag+ to form Ag3AsO4 as a precipitate. (c) If it took 25.0 mL of 0.102 M Ag+ to reach the equivalence point in this titration, what is the mass percentage of arsenic in the pesticide?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Titration and Equivalence Point
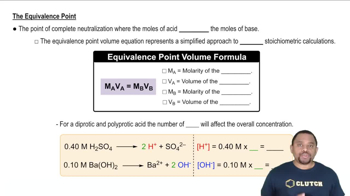
Stoichiometry

Mass Percentage

The arsenic in a 1.22-g sample of a pesticide was converted to AsO43- by suitable chemical treatment. It was then titrated using Ag+ to form Ag3AsO4 as a precipitate. (b) Name Ag3AsO4 by analogy to the corresponding compound containing phosphorus in place of arsenic.
Federal regulations set an upper limit of 50 parts per million (ppm) of NH3 in the air in a work environment [that is, 50 molecules of NH3(g) for every million molecules in the air]. Air from a manufacturing operation was drawn through a solution containing 1.00⨉102 mL of 0.0105 M HCl. The NH3 reacts with HCl according to: NH3(aq) + HCl(aq) → NH4Cl(aq). After drawing air through the acid solution for 10.0 min at a rate of 10.0 L/min, the acid was titrated. The remaining acid needed 13.1 mL of 0.0588 M NaOH to reach the equivalence point. (a) How many grams of NH3 were drawn into the acid solution?
