The accompanying photo shows the reaction between a solution of Cd(NO3)2 and one of Na2S. (b) What ions remain in solution?

Suppose you have a solution that might contain any or all of the following cations: Ni2+, Ag+, Sr2+, and Mn2+. Addition of HCl solution causes a precipitate to form. After filtering off the precipitate, H2SO4 solution is added to the resulting solution and another precipitate forms. This is filtered off, and a solution of NaOH is added to the resulting solution. No precipitate is observed. Which of the four ions listed above must be absent from the original solution?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
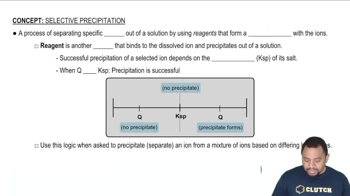
Precipitation Reactions
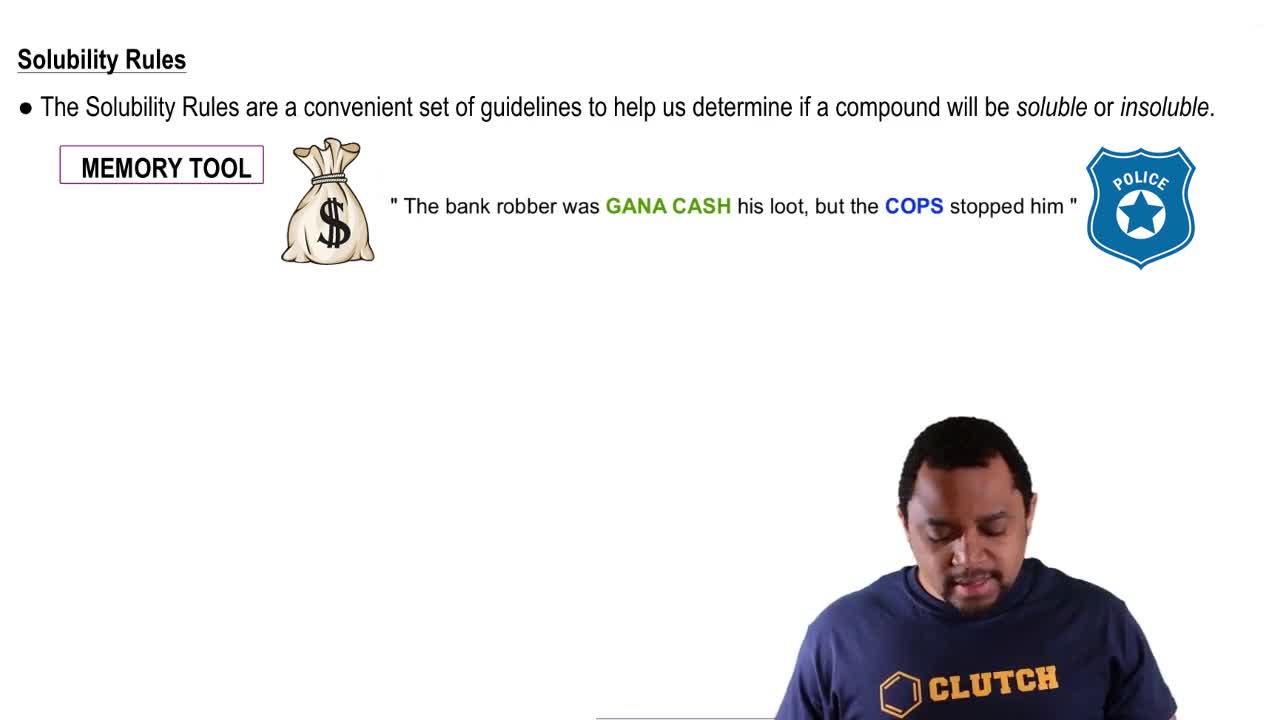
Solubility Rules
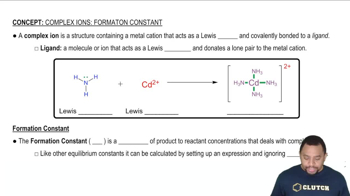
Complex Ion Formation
Uranium hexafluoride, UF6, is processed to produce fuel for nuclear reactors and nuclear weapons. UF6 can be produced in a two-step reaction. Solid uranium (IV) oxide, UO2, is first made to react with hydrofluoric acid (HF) solution to form solid UF4 with water as a by-product. UF4 further reacts with fluorine gas to form UF6. (a) Write the balanced molecular equations for the conversion of UO2 into UF4 and the conversion of UF4 to UF6. (b) Which step is an acid-base reaction?
The accompanying photo shows the reaction between a solution of Cd(NO3)2 and one of Na2S. (d) Is this a redox reaction?
Antacids are often used to relieve pain and promote healing in the treatment of mild ulcers. Write balanced net ionic equations for the reactions between the aqueous HCl in the stomach and each of the following substances used in various antacids: (a) Al(OH)3(s) (b) Mg(OH)2(s) (c) MgCO3(s) (d) NaAl(CO3)(OH)2(s) (e) CaCO3(s).
The commercial production of nitric acid involves the following chemical reactions:
4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g)
2 NO(g) + O2(g) → 2 NO2(g)
3 NO2(g) + H2O(l) → 2 HNO3(aq) + NO(g)
(a) Which of these reactions are redox reactions?
