We have learned in this chapter that many ionic solids dissolve in water as strong electrolytes; that is, as separated ions in solution. Which statement is most correct about this process? (a) Water is a strong acid and therefore is good at dissolving ionic solids. (b) Water is good at solvating ions because the hydrogen and oxygen atoms in water molecules bear partial charges. (c) The hydrogen and oxygen bonds of water are easily broken by ionic solids.
Ch.4 - Reactions in Aqueous Solution

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 4, Problem 17a
Ignoring protolysis reactions (i.e. proton transfer reaction), specify what ions are present in a solution upon dissolving each of the following substances in water: (a)Li2CO3
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the type of compound. Li2CO3 is an ionic compound, which means it will dissociate into its constituent ions when dissolved in water.
Step 2: Identify the ions that make up the compound. Li2CO3 is made up of Li+ (lithium ions) and CO32- (carbonate ions).
Step 3: Determine the number of each ion. The subscript '2' in Li2CO3 indicates that there are two Li+ ions for every one CO32- ion.
Step 4: Write the dissociation equation. When Li2CO3 is dissolved in water, it dissociates into 2 Li+ ions and 1 CO32- ion.
Step 5: Therefore, upon dissolving Li2CO3 in water, the ions present in the solution are Li+ and CO32-.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Dissociation of Ionic Compounds
When ionic compounds like Li2CO3 dissolve in water, they dissociate into their constituent ions. This process involves the separation of the positive and negative ions due to the interaction with water molecules, which stabilize the ions in solution.
Recommended video:
Guided course

Ionic Compounds Naming
Ionic Species in Solution
In the case of Li2CO3, the dissociation results in lithium ions (Li+) and carbonate ions (CO3^2-). Understanding the specific ions formed is crucial for predicting the behavior of the solution, including its conductivity and reactivity.
Recommended video:
Guided course
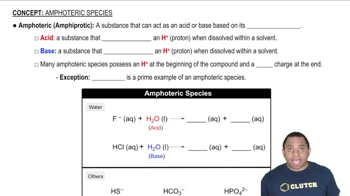
Amphoteric Species
Role of Water as a Solvent
Water is a polar solvent, meaning it has a partial positive charge on one side and a partial negative charge on the other. This polarity allows water to effectively solvate ions, facilitating their separation and stabilization in solution, which is essential for the dissolution process.
Recommended video:
Guided course
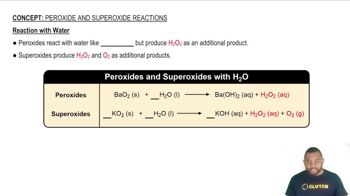
Reaction with Water
Related Practice
Textbook Question
1
views
Textbook Question
Would you expect that an anion would be physically closer to the oxygen or to the hydrogens of water molecules that surround it in solution?
3
views
Textbook Question
Ignoring protolysis reactions (i.e. proton transfer reaction), specify what ions are present in a solution upon dissolving each of the following substances in water: (b)(NH4)3PO4 (c) Na2Cr2O7 (d) NaPF6.
2
views
Textbook Question
Specify what ions are present upon dissolving each of the following substances in water: (a) MgI2
1
views
Textbook Question
Specify what ions are present upon dissolving each of the following substances in water: (b) K2CO3
