Federal regulations set an upper limit of 50 parts per million (ppm) of NH3 in the air in a work environment [that is, 50 molecules of NH3(g) for every million molecules in the air]. Air from a manufacturing operation was drawn through a solution containing 1.00⨉102 mL of 0.0105 M HCl. The NH3 reacts with HCl according to: NH3(aq) + HCl(aq) → NH4Cl(aq). After drawing air through the acid solution for 10.0 min at a rate of 10.0 L/min, the acid was titrated. The remaining acid needed 13.1 mL of 0.0588 M NaOH to reach the equivalence point. (a) How many grams of NH3 were drawn into the acid solution?
Ch.4 - Reactions in Aqueous Solution

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 4, Problem 115b
Federal regulations set an upper limit of 50 parts per million (ppm) of NH3 in the air in a work environment [that is, 50 molecules of NH3(g) for every million molecules in the air]. Air from a manufacturing operation was drawn through a solution containing 1.00⨉102 mL of 0.0105 M HCl. The NH3 reacts with HCl according to: NH3(aq) + HCl(aq) → NH4Cl(aq). After drawing air through the acid solution for 10.0 min at a rate of 10.0 L/min, the acid was titrated. The remaining acid needed 13.1 mL of 0.0588 M NaOH to reach the equivalence point. (b) How many ppm of NH3 were in the air? (Air has a density of 1.20 g/L and an average molar mass of 29.0 g/mol under the conditions of the experiment.)
 Verified step by step guidance
Verified step by step guidance1
Calculate the total volume of air drawn through the solution: \(10.0 \text{ L/min} \times 10.0 \text{ min} = 100.0 \text{ L}\).
Determine the initial moles of HCl in the solution: \(0.0105 \text{ M} \times 0.100 \text{ L} = 0.00105 \text{ mol HCl}\).
Calculate the moles of NaOH used in the titration: \(0.0588 \text{ M} \times 0.0131 \text{ L} = 0.00076908 \text{ mol NaOH}\).
Determine the moles of HCl that reacted with NH\(_3\): Subtract the moles of NaOH from the initial moles of HCl: \(0.00105 \text{ mol} - 0.00076908 \text{ mol} = 0.00028092 \text{ mol HCl}\).
Convert the moles of NH\(_3\) to ppm: Calculate the moles of air using its density and molar mass, then use the ratio of NH\(_3\) moles to air moles to find ppm.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Stoichiometry
Stoichiometry is the calculation of reactants and products in chemical reactions based on the balanced chemical equation. It allows us to determine the amount of substances consumed and produced in a reaction. In this case, understanding the stoichiometric relationship between NH<sub>3</sub> and HCl is crucial for calculating the concentration of NH<sub>3</sub> in the air after the reaction.
Recommended video:
Guided course

Stoichiometry Concept
Molarity and Dilution
Molarity (M) is a measure of concentration defined as the number of moles of solute per liter of solution. In this problem, the initial concentration of HCl and the volume used are essential for determining how much HCl reacted with NH<sub>3</sub>. Additionally, the concept of dilution is important when calculating the remaining concentration of HCl after the reaction, which is then used to find the amount of NH<sub>3</sub> present.
Recommended video:
Guided course
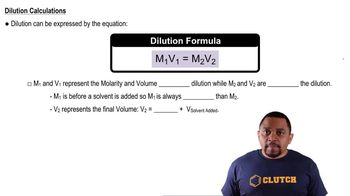
Dilution Equation
Parts Per Million (ppm)
Parts per million (ppm) is a unit of measurement used to describe the concentration of a substance in a solution or mixture. It indicates how many parts of a substance are present in one million parts of the total solution. In this question, converting the amount of NH<sub>3</sub> found in the air sample to ppm is necessary to assess compliance with federal regulations regarding air quality.
Recommended video:
Guided course
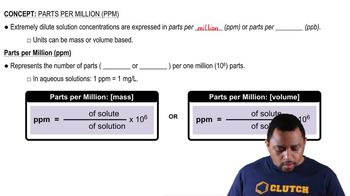
Parts per Million (ppm)
Related Practice
Textbook Question
Textbook Question
Potassium superoxide, KO2, is often used in oxygen masks (such as those used by firefighters) because KO2 reacts with CO2 to release molecular oxygen. Experiments indicate that 2 mol of KO2(s) react with each mole of CO2(g). (b) Indicate the oxidation number for each atom involved in the reaction in part (a). What elements are being oxidized and reduced?
Textbook Question
Chlorine dioxide gas 1ClO22 is used as a commercial bleachingagent. It bleaches materials by oxidizing them. In thecourse of these reactions, the ClO2 is itself reduced. (b) Why do you think that ClO2 is reduced so readily?
