
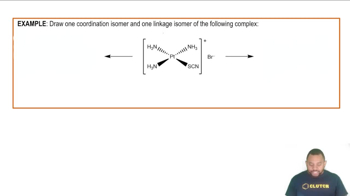
Section 2.9 introduced the idea of structural isomerism, with 1-propanol and 2-propanol as examples. Determine which of these properties would distinguish these two substances: (a) boiling point, (b) combustion analysis results, (c) molecular weight, (d) density at a given temperature and pressure. You can check on the properties of these two compounds in Wolfram Alpha (http://www.wolframalpha.com/) or the CRC Handbook of Chemistry and Physics.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Structural Isomerism
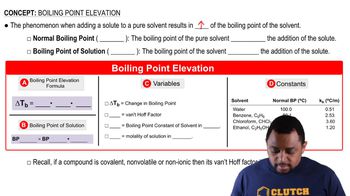
Boiling Point
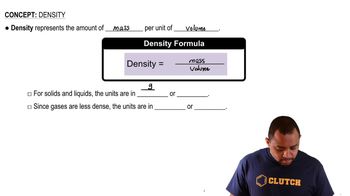
Density
(b) Because atoms are spherical, they cannot occupy all of the space of the cube. The silver atoms pack in the solid in such a way that 74% of the volume of the solid is actually filled with the silver atoms. Calculate the volume of a single silver atom.
Burning acetylene in oxygen can produce three different carbon-containing products: soot (very fine particles of graphite), CO(g), and CO2(g). (c) Why, when the oxygen supply is adequate, is CO2(g) the predominant carbon-containing product of the combustion of acetylene?
Hydrogen cyanide, HCN, is a poisonous gas. The lethal dose is approximately 300 mg HCN per kilogram of air when inhaled. (a) Calculate the amount of HCN that gives the lethal dose in a small laboratory room measuring 3.5 × 4.5 × 2.5 m. The density of air at 26 °C is 0.00118 g/cm3. (b) If the HCN is formed by reaction of NaCN with an acid such as H2SO4, what mass of NaCN gives the lethal dose in the room? 2 NaCN(s) + H2SO4(aq) → Na2SO4(aq) + 2 HCN(g)
Hydrogen cyanide, HCN, is a poisonous gas. The lethal dose is approximately 300 mg HCN per kilogram of air when inhaled. (c) HCN forms when synthetic fibers containing Orlon® or Acrilan ® burn. Acrilan® has an empirical formula of CH2CHCN, so HCN is 50.9% of the formula by mass. A rug measures 3.5 × 4.5 m and contains 850 g of Acrilan® fibers per square yard of carpet. If the rug burns, will a lethal dose of HCN be generated in the room? Assume that the yield of HCN from the fibers is 20% and that the carpet is 50% consumed.
