Calculate the percentage by mass of the indicated element in the following compounds: (e) oxygen in the insect pheromone sulcatol, C8H16O
Ch.3 - Chemical Reactions and Reaction Stoichiometry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 3, Problem 28a
Calculate the percentage of carbon by mass in each of the compounds represented by the following models: (a)
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the molecular formula of the compound. This information is usually given in the problem or can be determined from the model.
Step 2: Calculate the molar mass of the compound. This is done by adding up the atomic masses of all the atoms in the compound. The atomic masses can be found on the periodic table.
Step 3: Calculate the molar mass of the carbon in the compound. This is done by multiplying the atomic mass of carbon (approximately 12.01 g/mol) by the number of carbon atoms in the compound.
Step 4: Divide the molar mass of the carbon by the molar mass of the compound and multiply by 100 to get the percentage of carbon by mass in the compound.
Step 5: Repeat the process for each compound in the problem.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molar Mass
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). It is calculated by summing the atomic masses of all the atoms in a molecule. Understanding molar mass is essential for determining the mass percentage of an element in a compound, as it provides the necessary context for comparing the mass of the element to the total mass of the compound.
Recommended video:
Guided course

Molar Mass Concept
Mass Percentage
Mass percentage is a way of expressing the concentration of an element in a compound, calculated by dividing the mass of the element by the total mass of the compound and multiplying by 100. This concept is crucial for the question, as it directly relates to how to quantify the proportion of carbon in the given compounds, allowing for a clear comparison of elemental composition.
Recommended video:
Guided course

Mass Percent Calculation
Chemical Formulas
Chemical formulas represent the composition of a compound, indicating the types and numbers of atoms present. For example, in the formula C2H6, there are two carbon atoms and six hydrogen atoms. Understanding how to interpret chemical formulas is vital for identifying the elements involved and calculating their respective contributions to the overall mass of the compound.
Recommended video:
Guided course
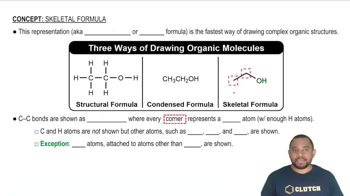
Skeletal Formula
Related Practice
Textbook Question
Textbook Question
Calculate the percentage by mass of the indicated element in the following compounds: (f) carbon in sucrose, C12H22O11, the compound that is responsible for the sweet taste of table sugar.
Textbook Question
Based on the following structural formulas, calculate the percentage of carbon by mass present in each compound: (a) Benzaldehyde (almond fragrance) (b) Vanillin (vanilla flavor) c) Isopentyl acetate (banana flavor)
Textbook Question
(a) Write 'true' or 'false' for each statement. (a) A mole of horses contain a mole of horse legs.
2
views
Textbook Question
(a) Write 'true' or 'false' for each statement. (a) A mole of horses contain a mole of horse legs.
Textbook Question
(a) Write 'true' or 'false' for each statement. (b) A mole of water has a mass of 18.0 g.
1
views
