Balance the following equations:
(a) CaS(s) + H2O(l) → Ca(HS2)2(aq) + Ca(OH)2(aq)
(b) NH3(g) + O2(g) → NO(g) + H2O(g)
(c) FeCl3(s) + Na2CO3(aq) → Fe2(CO3)3(s) + NaCl(aq)
(d) FeS2(s) + O2(g) → Fe2O3(s) + SO2(g)

 Verified step by step guidance
Verified step by step guidance

Balance the following equations:
(a) CaS(s) + H2O(l) → Ca(HS2)2(aq) + Ca(OH)2(aq)
(b) NH3(g) + O2(g) → NO(g) + H2O(g)
(c) FeCl3(s) + Na2CO3(aq) → Fe2(CO3)3(s) + NaCl(aq)
(d) FeS2(s) + O2(g) → Fe2O3(s) + SO2(g)
Balance the following equations: (a) CF4(l) + Br2(g) → CBr4(l) + F2(g) (b) Cu(s) + HNO3(aq) → Cu(NO3)2(aq) + NO2(g) + H2O(l)
Balance the following equations: (c) MnO2(s) + HCl(aq) → MnCl2(s) + H2O(l) + Cl2(g)
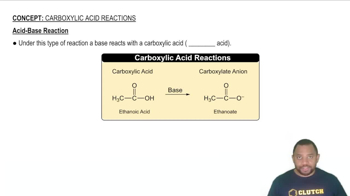
Write balanced chemical equations corresponding to each of the following descriptions: (a) Potassium cyanide reacts with an aqueous solution of sulfuric acid to form hydrogen cyanide gas. (b) When an aqueous solution of ammonium nitrite (NH4NO2) reacts with an aqueous solution of potassium hydroxide, ammonia gas, water and metal nitrate is formed. (c) When hydrogen gas is passed over solid hot iron(III) oxide, the resulting reaction produces iron and gaseous water. (d) When liquid ethanoic acid (CH3COOH) is combusted, carbon dioxide and water are formed.
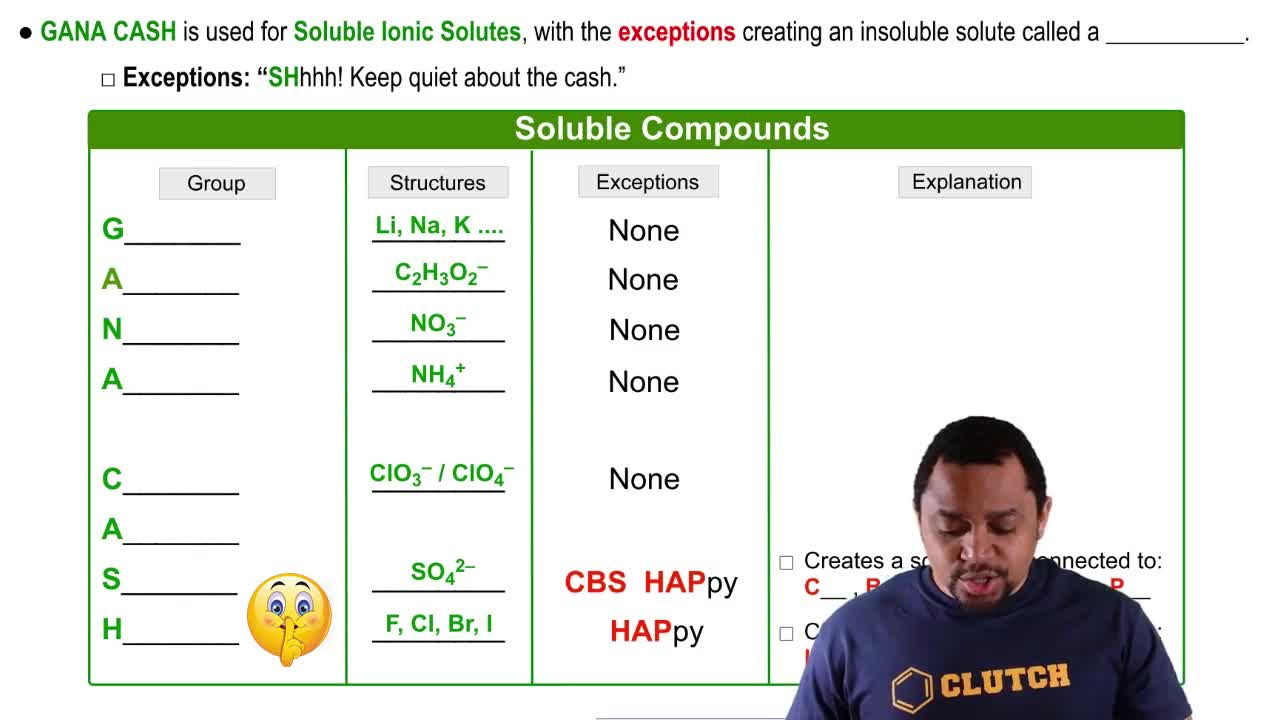
Write balanced chemical equations to correspond to each of the following descriptions: (a) When sulfur trioxide gas reacts with water, a solution of sulfuric acid forms. (b) Boron sulfide, B2S3(s), reacts violently with water to form dissolved boric acid, H3BO3, and hydrogen sulfide gas.
Write balanced chemical equations to correspond to each of the following descriptions: (c) Phosphine, PH3(g), combusts in oxygen gas to form water vapor and solid tetraphosphorus decaoxide.