The fat stored in a camel's hump is a source of both energy and water. Calculate the mass of H2O produced by the metabolism of 1.0 kg of fat, assuming the fat consists entirely of tristearin 1C57H110O62, a typical animal fat, and assuming that during metabolism, tristearin reacts with O2 to form only CO2 and H2O.
Ch.3 - Chemical Reactions and Reaction Stoichiometry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 3, Problem 104
A mixture containing KClO3, K2CO3, KHCO3, and KCl was heated, producing CO2, O2, and H2O gases according to the following equations: 2 KClO31s2¡2 KCl1s2 + 3 O21g2 2 KHCO31s2¡K2O1s2 + H2O1g2 + 2 CO21g2 K2CO31s2¡K2O1s2 + CO21g2 The KCl does not react under the conditions of the reaction. If 100.0 g of the mixture produces 1.80 g of H2O, 13.20 g of CO2, and 4.00 g of O2, what was the composition of the original mixture? (Assume complete decomposition of the mixture.) How many grams of K2CO3 were in the original mixture?
 Verified step by step guidance
Verified step by step guidance1
Identify the chemical reactions involved and the products formed: \(2 \text{KClO}_3 \rightarrow 2 \text{KCl} + 3 \text{O}_2\), \(2 \text{KHCO}_3 \rightarrow \text{K}_2\text{O} + \text{H}_2\text{O} + 2 \text{CO}_2\), \(\text{K}_2\text{CO}_3 \rightarrow \text{K}_2\text{O} + \text{CO}_2\).
Calculate the moles of each gas produced using their respective molar masses: \(\text{H}_2\text{O}\), \(\text{CO}_2\), and \(\text{O}_2\).
Use stoichiometry to determine the moles of each reactant that decomposed to produce the given moles of gases.
Convert the moles of each reactant back to grams using their molar masses to find the mass of each component in the original mixture.
Subtract the masses of \(\text{KClO}_3\), \(\text{KHCO}_3\), and \(\text{K}_2\text{CO}_3\) from the total mass of the mixture to find the mass of \(\text{KCl}\).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
7mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Stoichiometry
Stoichiometry is the calculation of reactants and products in chemical reactions based on the conservation of mass. It involves using balanced chemical equations to determine the relationships between the amounts of substances consumed and produced. In this question, stoichiometry is essential for relating the masses of the gases produced to the amounts of the original compounds in the mixture.
Recommended video:
Guided course

Stoichiometry Concept
Gas Laws
Gas laws describe the behavior of gases in relation to pressure, volume, temperature, and the number of moles. Understanding these laws helps in calculating the expected volumes or masses of gases produced in a reaction. In this scenario, the production of gases like CO2, O2, and H2O must be analyzed to determine the original composition of the solid mixture.
Recommended video:
Guided course
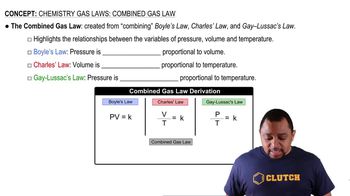
Combined Gas Law
Decomposition Reactions
Decomposition reactions involve the breakdown of a compound into simpler substances, often producing gases. In this question, the decomposition of KClO3, KHCO3, and K2CO3 is crucial to understanding how the original mixture generates the observed gases. Recognizing the specific products formed from each compound allows for the calculation of their initial amounts in the mixture.
Recommended video:
Guided course
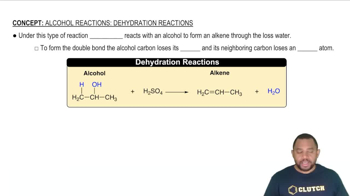
Alcohol Reactions: Dehydration Reactions
Related Practice
Textbook Question
Textbook Question
A mixture of N21g2 and H21g2 reacts in a closed container to form ammonia, NH31g2. The reaction ceases before either reactant has been totally consumed. At this stage 3.0 mol N2, 3.0 mol H2, and 3.0 mol NH3 are present. How many moles of N2 and H2 were present originally?
Textbook Question
When a mixture of 10.0 g of acetylene (C2H2) and 10.0 g of oxygen (O2) is ignited, the resulting combustion reaction produces CO2 and H2O. (c) How many grams of CO2 and H2O are present after the reaction is complete?
Textbook Question
When a mixture of 10.0 g of acetylene (C2H2) and 10.0 g of oxygen (O2) is ignited, the resulting combustion reaction produces CO2 and H2O. (c) How many grams of C2H2 are present after the reaction is complete?
