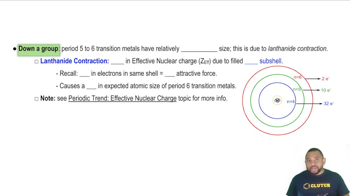
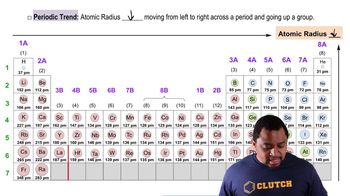
Textbook Question
Which of these crystal-field splitting diagrams represents:
a. a weak-field octahedral complex of Fe³⁺ ,
b. a strong-field octahedral complex of Fe³⁺
c. a tetrahedral complex of Fe³⁺
d. a tetrahedral complex of Ni²⁺ (The diagrams do not indicate the relative magnitudes of ∆. ) [Find more in Section 23.6.]