Textbook Question
The lobes of which d orbitals point directly between the ligands in b. tetrahedral geometry?

 Verified step by step guidance
Verified step by step guidance
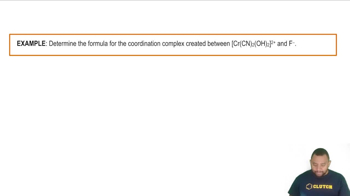

The lobes of which d orbitals point directly between the ligands in b. tetrahedral geometry?
As shown in Figure 23.26, the d-d transition of [Ti(H2O)6]³⁺ produces an absorption maximum at a wavelength of about 500 nm .
a. What is the magnitude of ∆ for [Ti(H2O)6]³⁺ in kJ/mol?
b. How would the magnitude of ∆ change if the H2O ligands in [Ti(H2O)6]]³⁺ were placed with NH3 ligands?
A classmate says, “A weak-field ligand usually means the complex is high spin.” Is your classmate correct? Explain.
For a given metal ion and set of ligands, is the crystal-field splitting energy larger for a tetrahedral or an octahedral geometry?