Textbook Question
Complete the exercises below. Draw the crystal-field energy-level diagrams and show the placement of electrons for each of the following complexes:
a. [VCl6]3–,
b. [FeF6]3– (a high-spin complex),

 Verified step by step guidance
Verified step by step guidance

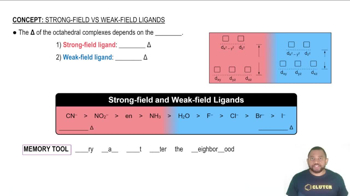
Complete the exercises below. Draw the crystal-field energy-level diagrams and show the placement of electrons for each of the following complexes:
a. [VCl6]3–,
b. [FeF6]3– (a high-spin complex),
Draw the crystal-field energy-level diagrams and show the placement of electrons for each of the following complexes:
d. [NiCl4]2+ (tetrahedral),