Ch.23 - Transition Metals and Coordination Chemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 23, Problem 46
Complete the exercises below. Determine if each of the following metal complexes is chiral and therefore has an optical isomer: a. square planar [Pd(en)(CN)₂].
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of chirality in coordination complexes. Chirality occurs when a molecule cannot be superimposed on its mirror image, often due to the presence of a chiral center.
Step 2: Identify the geometry of the complex. The given complex [Pd(en)(CN)₂] is described as square planar, which is a common geometry for d⁸ metal ions like Pd(II).
Step 3: Analyze the ligands in the complex. The complex contains ethylenediamine (en), a bidentate ligand, and two cyanide (CN⁻) ligands. Ethylenediamine can potentially introduce chirality due to its ability to form chelate rings.
Step 4: Consider the symmetry of the complex. In a square planar complex, the arrangement of ligands around the central metal ion is typically symmetric. Check if the arrangement of ligands allows for a non-superimposable mirror image.
Step 5: Determine if the complex is chiral. For a square planar complex to be chiral, it must lack a plane of symmetry. Evaluate the spatial arrangement of the ligands to see if such a plane exists.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Chirality
Chirality refers to the geometric property of a molecule that makes it non-superimposable on its mirror image, much like left and right hands. A chiral molecule typically has at least one chiral center, often a carbon atom bonded to four different substituents. In coordination chemistry, chirality can arise from the arrangement of ligands around a central metal atom, leading to distinct optical isomers.
Recommended video:
Guided course
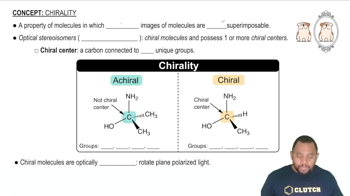
Chirality
Coordination Geometry
Coordination geometry describes the spatial arrangement of ligands around a central metal ion in a complex. Common geometries include octahedral, tetrahedral, and square planar. The geometry influences the symmetry of the complex, which is crucial for determining whether the complex can exist as chiral isomers.
Recommended video:
Guided course
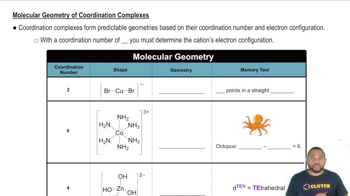
Molecular Geometry of Coordination Complexes
Optical Isomerism
Optical isomerism is a form of stereoisomerism where isomers differ in the way they rotate plane-polarized light. Chiral compounds can exist as two enantiomers, which are mirror images of each other and have different optical activities. The presence of optical isomers is significant in many fields, including pharmaceuticals, where different enantiomers can have different biological effects.
Recommended video:
Guided course
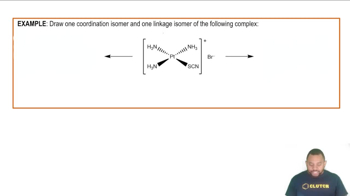
Isomerism in Coordination Complexes Example
Related Practice
