Ch.23 - Transition Metals and Coordination Chemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 23, Problem 44
Complete the exercises below. Determine if each of the following complexes exhibits geometric isomerism. If geometric isomers exist, determine how many there are. c. Square-planar [Pd(en)(CN)₂].
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the structure of the complex. The complex [Pd(en)(CN)_2] is square-planar, which means it has a central palladium (Pd) atom with four ligands arranged in a square plane around it.
Step 2: Identify the ligands. In this complex, 'en' stands for ethylenediamine, a bidentate ligand, and 'CN' stands for cyanide, a monodentate ligand. Ethylenediamine can bind to the metal at two points, while each cyanide ion binds at one point.
Step 3: Consider the possible arrangements of the ligands. In a square-planar complex, the ligands can be arranged in different ways around the central metal atom. Check if the ligands can be arranged such that different spatial arrangements (geometric isomers) are possible.
Step 4: Determine the possible geometric isomers. For square-planar complexes, geometric isomerism can occur if there are different ways to arrange the ligands around the central metal. Consider the positions of the bidentate ligand (en) and the monodentate ligands (CN) to see if different configurations are possible.
Step 5: Count the number of geometric isomers. If different spatial arrangements are possible, count how many distinct configurations exist. This will give you the number of geometric isomers for the complex.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Geometric Isomerism
Geometric isomerism occurs when compounds have the same molecular formula and connectivity of atoms but differ in the spatial arrangement of their atoms. This type of isomerism is particularly relevant in coordination complexes, where ligands can occupy different positions around a central metal ion, leading to distinct isomers that may have different physical and chemical properties.
Recommended video:
Guided course
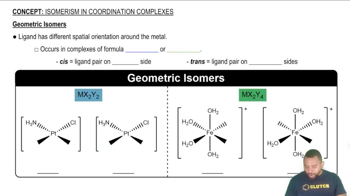
Geometric Isomers
Square-Planar Geometry
Square-planar geometry is a molecular shape that occurs when a central atom is surrounded by four ligands arranged at the corners of a square, typically seen in d8 metal complexes like palladium(II). In this geometry, the ligands are positioned 90 degrees apart in the same plane, which allows for the possibility of geometric isomers based on the relative positions of the ligands.
Recommended video:
Guided course
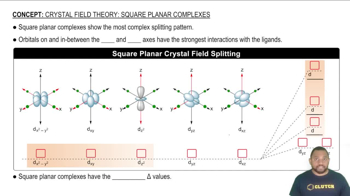
Square planar complexes show the most complex splitting pattern.
Ligand Types and Coordination
Ligands are ions or molecules that can donate a pair of electrons to a central metal atom to form a coordination complex. In the case of [Pd(en)(CN)₂], 'en' (ethylenediamine) is a bidentate ligand that binds through two donor atoms, while 'CN' (cyanide) is a monodentate ligand. The arrangement of these ligands around the palladium center influences the potential for geometric isomerism, as different spatial arrangements can lead to distinct isomers.
Recommended video:
Guided course
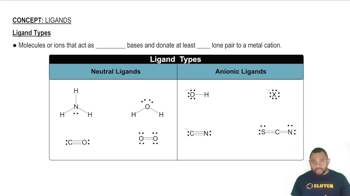
Ligand Types
Related Practice
