Ch.23 - Transition Metals and Coordination Chemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 23, Problem 24
Complete the exercises below. Crystals of hydrated chromium(III) chloride are green, have an empirical formula of CrCl₃ • 6H₂O, and are highly soluble. a. Write the complex ion that exists in this compound. b. If the complex is treated with excess AgNO₃ (aq), how many moles of AgCl will precipitate per mole of CrCl₃ • 6H₂O dissolved in solution? c. Crystals of anhydrous chromium(III) chloride are violet and insoluble in aqueous solution. The coordination geometry of chromium in these crystals is octahedral, as is almost always the case for Cr³⁺. How can this be the case if the ratio of Cr to Cl is not 1:6?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the complex ion in the hydrated chromium(III) chloride. The empirical formula is CrCl₃ • 6H₂O, which suggests that the chromium ion is coordinated with water molecules. The complex ion can be written as [Cr(H₂O)₆]³⁺, where chromium is surrounded by six water molecules.
Step 2: Determine the number of chloride ions outside the coordination sphere. In CrCl₃ • 6H₂O, the three chloride ions are not part of the coordination sphere and are free to dissociate in solution.
Step 3: Calculate the moles of AgCl precipitated. When the complex is treated with excess AgNO₃, the free chloride ions will react with Ag⁺ ions to form AgCl. Since there are three chloride ions per formula unit of CrCl₃ • 6H₂O, three moles of AgCl will precipitate per mole of CrCl₃ • 6H₂O.
Step 4: Explain the coordination geometry of anhydrous chromium(III) chloride. In the anhydrous form, CrCl₃, the chromium ion is still in an octahedral geometry, but the chloride ions are directly coordinated to the chromium, forming a network solid that is insoluble in water.
Step 5: Discuss the difference in solubility and color between hydrated and anhydrous forms. The hydrated form is green and soluble due to the presence of water molecules in the coordination sphere, while the anhydrous form is violet and insoluble because the chloride ions are directly bonded to chromium, forming a stable lattice.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Coordination Complexes
Coordination complexes consist of a central metal atom or ion bonded to surrounding molecules or ions, known as ligands. In the case of chromium(III) chloride, the Cr³⁺ ion coordinates with water molecules, forming a complex ion. Understanding the nature of these complexes is crucial for predicting their behavior in reactions, such as precipitation with silver nitrate.
Recommended video:
Guided course
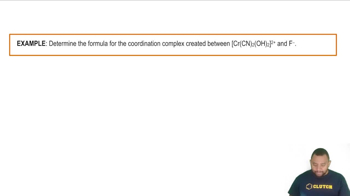
Coordination Complexes Example
Precipitation Reactions
Precipitation reactions occur when two soluble salts react in solution to form an insoluble product, or precipitate. In this scenario, when excess AgNO₃ is added to the solution containing the chromium complex, Ag⁺ ions react with Cl⁻ ions to form AgCl, which precipitates out of the solution. The stoichiometry of the reaction helps determine the amount of precipitate formed.
Recommended video:
Guided course

Selective Precipitation
Coordination Geometry
Coordination geometry refers to the spatial arrangement of ligands around a central metal ion in a coordination complex. For Cr³⁺, the common geometry is octahedral, which can occur even if the metal-to-ligand ratio is not 1:6. This is because multiple ligands can coordinate to the metal ion, allowing for different structural arrangements while maintaining octahedral coordination.
Recommended video:
Guided course
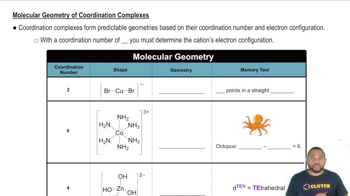
Molecular Geometry of Coordination Complexes
Related Practice
