Ch.22 - Chemistry of the Nonmetals

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 22, Problem 74
Speculate on why carbon forms carbonate rather than silicate analogs.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the basic chemistry of carbon and silicon. Carbon and silicon are both in Group 14 of the periodic table, meaning they have similar valence electron configurations, but they differ significantly in their chemical behavior.
Step 2: Consider the size and electronegativity of carbon versus silicon. Carbon is smaller and more electronegative than silicon, which affects the types of bonds they form. Carbon tends to form strong covalent bonds, while silicon can form more ionic character bonds.
Step 3: Examine the stability of carbonates versus silicates. Carbonates (CO₃²⁻) are stable due to the resonance stabilization of the carbonate ion, where the negative charge is delocalized over the three oxygen atoms. Silicates, on the other hand, form complex structures with silicon-oxygen bonds that are more covalent and less prone to resonance stabilization.
Step 4: Analyze the role of hybridization in carbon compounds. Carbon readily undergoes sp² hybridization to form planar structures like carbonate ions, which is energetically favorable. Silicon, due to its larger size, does not hybridize in the same way and forms tetrahedral structures in silicates.
Step 5: Consider the environmental and biological context. Carbonates are prevalent in biological systems and the Earth's crust due to their solubility and ability to form stable minerals like calcium carbonate. Silicates are more common in geological formations but do not play a significant role in biological systems.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Bonding and Structure
Carbon forms carbonate (CO3^2-) due to its ability to form strong covalent bonds with oxygen, resulting in a stable trigonal planar structure. This geometry allows for effective overlap of atomic orbitals, leading to resonance stabilization. In contrast, silicon, while also capable of forming silicates, has a larger atomic radius and different bonding characteristics that result in less stable structures compared to carbonates.
Recommended video:
Guided course
Chemical Bonds
Electronegativity and Ionic Character
Carbon has a moderate electronegativity, allowing it to form stable covalent bonds with oxygen in carbonate ions. The difference in electronegativity between carbon and oxygen facilitates the formation of polar covalent bonds, which contribute to the stability of the carbonate ion. Silicon, being less electronegative, tends to form more ionic bonds in silicates, which can lead to less stable structures in comparison to carbonates.
Recommended video:
Guided course
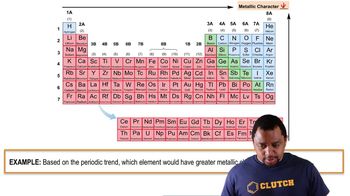
Metallic Character Example
Acid-Base Chemistry
Carbonate ions can act as weak bases, reacting with acids to form carbon dioxide and water. This property is crucial in biological and geological processes, such as buffering systems in natural waters. Silicates, on the other hand, do not exhibit the same level of acid-base behavior, limiting their role in similar chemical equilibria, which further emphasizes the unique stability and reactivity of carbonates compared to silicates.
Recommended video:
Guided course

Arrhenius Acids and Bases
Related Practice
