Ch.22 - Chemistry of the Nonmetals

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 22, Problem 56
Complete the exercises below. Write complete balanced half-reactions for: a. reduction of nitrate ion to NO in acidic solution, b. oxidation of HNO₂ to NO₂ in acidic solution.
 Verified step by step guidance
Verified step by step guidance1
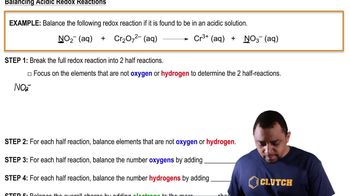
Step 1: Identify the species involved in each half-reaction. For part (a), the nitrate ion (NO₃⁻) is reduced to nitric oxide (NO). For part (b), nitrous acid (HNO₂) is oxidized to nitrogen dioxide (NO₂).
Step 2: Write the unbalanced half-reactions. For part (a), NO₃⁻ → NO. For part (b), HNO₂ → NO₂.
Step 3: Balance the atoms other than oxygen and hydrogen. In both reactions, nitrogen is already balanced.
Step 4: Balance the oxygen atoms by adding H₂O molecules. For part (a), add H₂O to the right side to balance the oxygen atoms. For part (b), add H₂O to the left side.
Step 5: Balance the hydrogen atoms by adding H⁺ ions. For part (a), add H⁺ to the left side. For part (b), add H⁺ to the right side.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Half-Reactions
Half-reactions are equations that show either the reduction or oxidation process occurring in a redox reaction. They are essential for balancing redox reactions, as they separate the two processes, allowing for a clearer understanding of electron transfer. Each half-reaction includes the species being oxidized or reduced, along with electrons, protons, and any other necessary ions to balance the charge and mass.
Recommended video:
Guided course
Redox Half Reactions Example
Acidic Solution
An acidic solution is one where the concentration of hydrogen ions (H⁺) is higher than that of hydroxide ions (OH⁻). In redox reactions occurring in acidic conditions, protons are often added to balance the charge in half-reactions. Understanding the role of H⁺ ions is crucial for accurately writing balanced half-reactions, especially when dealing with species that change oxidation states.
Recommended video:
Guided course

Solution Components
Oxidation States
Oxidation states (or oxidation numbers) indicate the degree of oxidation of an atom in a compound. They help in identifying which species is oxidized and which is reduced in a redox reaction. By determining the oxidation states of the reactants and products, one can write balanced half-reactions that reflect the transfer of electrons, ensuring that the overall charge and mass are conserved.
Recommended video:
Guided course

Oxidation Numbers
Related Practice
