Ch.22 - Chemistry of the Nonmetals

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 22, Problem 67
Complete the exercises below. Write a balanced equation for each of the following reactions: a. Hydrogen cyanide is formed commercially by passing a mixture of methane, ammonia, and air over a catalyst at 800 °C. Water is a by-product of the reaction. b. Baking soda reacts with acids to produce carbon dioxide gas. c. When barium carbonate reacts in air with sulfur dioxide, barium sulfate and carbon dioxide form.
 Verified step by step guidance
Verified step by step guidance1
Identify the reactants and products for each reaction. For reaction (a), the reactants are methane (CH_4), ammonia (NH_3), and oxygen (O_2) from air, and the products are hydrogen cyanide (HCN) and water (H_2O).
Write the unbalanced chemical equation for reaction (a): CH_4 + NH_3 + O_2 → HCN + H_2O.
Balance the chemical equation for reaction (a) by adjusting the coefficients to ensure the same number of each type of atom on both sides of the equation.
For reaction (b), identify the reactants and products: baking soda (NaHCO_3) reacts with an acid (e.g., HCl) to produce carbon dioxide (CO_2), water (H_2O), and a salt (e.g., NaCl).
Write and balance the chemical equation for reaction (b): NaHCO_3 + HCl → CO_2 + H_2O + NaCl.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Balancing Chemical Equations
Balancing chemical equations is the process of ensuring that the number of atoms for each element is the same on both the reactant and product sides of the equation. This is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. A balanced equation provides a clear representation of the reactants and products involved, allowing for accurate stoichiometric calculations.
Recommended video:
Guided course

Balancing Chemical Equations
Types of Chemical Reactions
Understanding the types of chemical reactions is essential for predicting the products of a reaction. Common types include synthesis, decomposition, single replacement, and double replacement reactions. For example, the reaction of baking soda with acids is a classic acid-base reaction that produces carbon dioxide gas, while the reaction of barium carbonate with sulfur dioxide involves a combination of a carbonate and a sulfur dioxide, leading to the formation of sulfate and carbon dioxide.
Recommended video:
Guided course
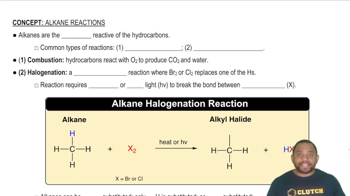
Common Types of Alkane Reactions
Catalysts in Chemical Reactions
A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. In the context of the first reaction, a catalyst is used to facilitate the formation of hydrogen cyanide from methane, ammonia, and air at high temperatures. Catalysts work by lowering the activation energy required for the reaction to occur, allowing the reaction to proceed more efficiently and at lower temperatures.
Recommended video:
Guided course
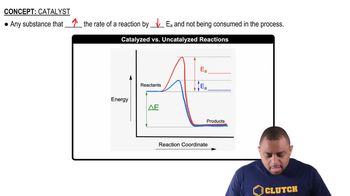
Catalyzed vs. Uncatalyzed Reactions
Related Practice
