Ch.22 - Chemistry of the Nonmetals

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 22, Problem 62
Complete the exercises below. Write a balanced equation for each of the following reactions: a. hydrolysis of PCl₅ b. dehydration of phosphoric acid (also called orthophosphoric acid) to form pyrophosphoric acid.
 Verified step by step guidance
Verified step by step guidance1
Identify the reactants and products for the hydrolysis of PCl₅. Hydrolysis involves the reaction of a compound with water. For PCl₅, the reaction with water will produce phosphoric acid (H₃PO₄) and hydrochloric acid (HCl).
Write the unbalanced chemical equation for the hydrolysis of PCl₅: \( \text{PCl}_5 + \text{H}_2\text{O} \rightarrow \text{H}_3\text{PO}_4 + \text{HCl} \).
Balance the chemical equation by ensuring the number of each type of atom is the same on both sides of the equation. Start by balancing the phosphorus atoms, then chlorine, hydrogen, and finally oxygen atoms.
For the dehydration of phosphoric acid, identify the reactants and products. Dehydration involves the removal of water. Orthophosphoric acid (H₃PO₄) will lose water to form pyrophosphoric acid (H₄P₂O₇).
Write the balanced chemical equation for the dehydration of phosphoric acid: \( 2\text{H}_3\text{PO}_4 \rightarrow \text{H}_4\text{P}_2\text{O}_7 + \text{H}_2\text{O} \).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Balancing Chemical Equations
Balancing chemical equations involves ensuring that the number of atoms of each element is the same on both sides of the equation. This is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. To balance an equation, coefficients are adjusted in front of the chemical formulas to achieve equal atom counts.
Recommended video:
Guided course

Balancing Chemical Equations
Hydrolysis Reactions
Hydrolysis reactions involve the reaction of a compound with water, leading to the breakdown of that compound. In the case of PCl₅, hydrolysis results in the formation of phosphoric acid and hydrochloric acid. Understanding the reactants and products of hydrolysis is crucial for writing the balanced equation accurately.
Recommended video:
Guided course
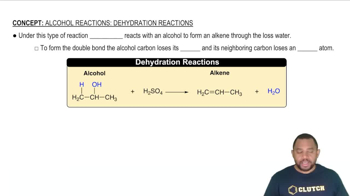
Alcohol Reactions: Dehydration Reactions
Dehydration Reactions
Dehydration reactions involve the removal of water from a compound, often resulting in the formation of a new compound. In the dehydration of phosphoric acid to form pyrophosphoric acid, a water molecule is eliminated. Recognizing the reactants and the nature of the reaction is essential for constructing the correct balanced equation.
Recommended video:
Guided course

Alcohol Reactions: Dehydration Reactions
Related Practice
