Ch.22 - Chemistry of the Nonmetals

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 22, Problem 14
Complete the exercises below. Consider the elements Li, K, Cl, C, Ne, and Ar. From this list, select the element that a. is most electronegative, b. has the greatest metallic character, c. most readily forms a positive ion, d. has the smallest atomic radius, e. forms π bonds most readily, f. has multiple allotropes.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the most electronegative element. Electronegativity increases across a period and decreases down a group in the periodic table. Compare the elements Li, K, Cl, C, Ne, and Ar to determine which is most electronegative.
Step 2: Determine the element with the greatest metallic character. Metallic character increases down a group and decreases across a period. Analyze the given elements to find the one with the highest metallic character.
Step 3: Find the element that most readily forms a positive ion. Elements that easily lose electrons to form cations are typically metals. Consider the elements and their positions in the periodic table to identify which forms a positive ion most readily.
Step 4: Identify the element with the smallest atomic radius. Atomic radius decreases across a period and increases down a group. Compare the atomic radii of the elements to find the smallest one.
Step 5: Determine which element forms π bonds most readily and has multiple allotropes. Elements that can form π bonds typically have p orbitals available for bonding, and those with multiple allotropes can exist in different structural forms. Analyze the elements to identify which fits these criteria.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electronegativity
Electronegativity is a measure of an atom's ability to attract and hold onto electrons in a chemical bond. It varies across the periodic table, generally increasing from left to right and decreasing from top to bottom. Elements like fluorine are highly electronegative, while metals tend to have lower values. Understanding electronegativity helps predict bond types and molecular behavior.
Recommended video:
Guided course

Electronegativity Trends
Metallic Character
Metallic character refers to the set of properties associated with metals, including conductivity, malleability, ductility, and the tendency to lose electrons and form positive ions. This character increases down a group in the periodic table and decreases across a period. Recognizing metallic character is essential for understanding reactivity and bonding in elements.
Recommended video:
Guided course
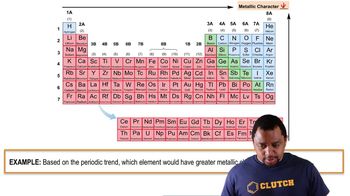
Metallic Character Example
Atomic Radius
Atomic radius is the distance from the nucleus of an atom to the outermost shell of electrons. It generally decreases across a period due to increased nuclear charge pulling electrons closer, and increases down a group as additional electron shells are added. The atomic radius is crucial for understanding trends in reactivity, ionization energy, and the formation of bonds.
Recommended video:
Guided course
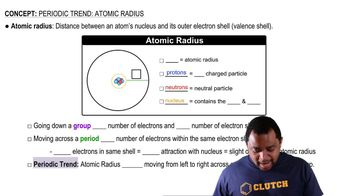
Atomic Radius
Related Practice
