Ch.22 - Chemistry of the Nonmetals

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 22, Problem 44
Complete the exercises below. An aqueous solution of SO₂ reduces a. aqueous KMnO₄ to MnSO₄ (aq) b. acidic aqueous K₂Cr₂O₇ to aqueous Cr⁵⁺, c. aqueous Hg₂(NO₃)₂ to mercury metal. Write balanced equations for these reactions.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the oxidation states of the elements involved in each reaction. For example, in KMnO₄, Mn is in the +7 oxidation state, and in MnSO₄, Mn is in the +2 oxidation state.
Step 2: Write the half-reaction for the reduction process. For example, for KMnO₄ to MnSO₄, the reduction half-reaction is: \( \text{MnO}_4^- + 8\text{H}^+ + 5\text{e}^- \rightarrow \text{Mn}^{2+} + 4\text{H}_2\text{O} \).
Step 3: Write the half-reaction for the oxidation process. For SO₂, it is oxidized to sulfate ion (SO₄²⁻): \( \text{SO}_2 + 2\text{H}_2\text{O} \rightarrow \text{SO}_4^{2-} + 4\text{H}^+ + 2\text{e}^- \).
Step 4: Balance the electrons between the oxidation and reduction half-reactions. Multiply the half-reactions by appropriate coefficients to equalize the number of electrons transferred.
Step 5: Combine the balanced half-reactions to form the overall balanced equation for each reaction. Ensure that all atoms and charges are balanced in the final equation.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Redox Reactions
Redox reactions, or reduction-oxidation reactions, involve the transfer of electrons between species. In these reactions, one substance is oxidized (loses electrons) while another is reduced (gains electrons). Understanding the oxidation states of the elements involved is crucial for identifying which species undergo these changes, as well as for balancing the overall reaction.
Recommended video:
Guided course
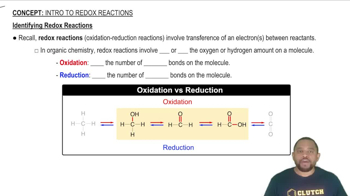
Identifying Redox Reactions
Balancing Chemical Equations
Balancing chemical equations is the process of ensuring that the number of atoms for each element is the same on both sides of the equation. This is essential for obeying the law of conservation of mass. Techniques such as the half-reaction method can be particularly useful in redox reactions, where separate oxidation and reduction half-reactions are balanced before combining them into a complete equation.
Recommended video:
Guided course

Balancing Chemical Equations
Acid-Base Chemistry
Acid-base chemistry involves the transfer of protons (H⁺ ions) between reactants. In the context of the given reactions, acidic conditions can influence the oxidation states of certain elements, such as chromium in K₂Cr₂O₇. Recognizing the role of acids and bases in redox reactions is important for predicting the products and balancing the equations correctly.
Recommended video:
Guided course

Arrhenius Acids and Bases
Related Practice
