Each of the following nuclei undergoes either beta decay or positron emission. Predict the type of emission for each: (b) 8938Sr.
Ch.21 - Nuclear Chemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 21, Problem 23
Which of the following nuclides have magic numbers of both protons and neutrons: (a) helium-4, (b) oxygen-18, (c) calcium-40, (d) zinc-66, (e) lead-208?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of magic numbers in nuclear physics. Magic numbers are specific numbers of protons or neutrons in a nucleus that result in a more stable configuration. These numbers are 2, 8, 20, 28, 50, 82, and 126.
Step 2: Identify the number of protons and neutrons in each nuclide. For example, helium-4 has 2 protons and 2 neutrons, oxygen-18 has 8 protons and 10 neutrons, calcium-40 has 20 protons and 20 neutrons, zinc-66 has 30 protons and 36 neutrons, and lead-208 has 82 protons and 126 neutrons.
Step 3: Compare the number of protons and neutrons in each nuclide to the list of magic numbers. Check if both the number of protons and the number of neutrons are magic numbers.
Step 4: Determine which nuclides have both protons and neutrons that match the magic numbers. For example, helium-4 has 2 protons and 2 neutrons, both of which are magic numbers.
Step 5: Conclude which nuclides have magic numbers for both protons and neutrons based on your comparison.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Magic Numbers
Magic numbers refer to specific numbers of protons or neutrons in a nucleus that result in a more stable configuration. These numbers are associated with complete shells of nucleons, similar to electron shells in atoms. The known magic numbers are 2, 8, 20, 28, 50, 82, and 126, which indicate particularly stable isotopes.
Recommended video:
Guided course
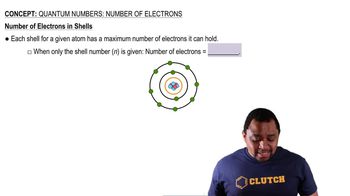
Number of Electrons in Shells
Nuclear Stability
Nuclear stability is determined by the balance between the attractive strong nuclear force and the repulsive electromagnetic force among protons. Nuclides with magic numbers of protons and neutrons tend to be more stable due to their complete nuclear shells, which minimizes energy and reduces the likelihood of radioactive decay.
Recommended video:
Guided course
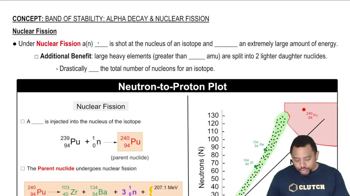
Band of Stability: Nuclear Fission
Isotopes and Nuclides
Isotopes are variants of a chemical element that have the same number of protons but different numbers of neutrons, resulting in different mass numbers. A nuclide is a specific isotope characterized by its number of protons and neutrons. Understanding isotopes is crucial for identifying which nuclides possess magic numbers and their implications for nuclear stability.
Recommended video:
Guided course
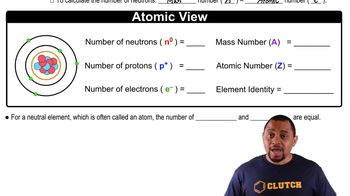
Isotopes
Related Practice
Textbook Question
1
views
Textbook Question
Despite the similarities in the chemical reactivity of elements in the lanthanide series, their abundances in Earth's crust vary by two orders of magnitude. This graph shows the relative abundance as a function of atomic number. Which of the following statements best explains the sawtooth variation across the series? (a) The elements with an odd atomic number lie above the belt of stability. (b) The elements with an odd atomic number lie below the belt of stability. (c) The elements with an even atomic number have a magic number of protons. (d) Pairs of protons have a special stability.
2
views
Textbook Question
Which of the following nuclides would you expect to be radioactive: 5826Fe, 6027Co, 9241Nb, mercury-202, radium-226? Justify your choices.
1
views
