The cloth shroud from around a mummy is found to have a 14C activity of 9.7 disintegrations per minute per gram of carbon as compared with living organisms that undergo 16.3 disintegrations per minute per gram of carbon. From the half-life for 14C decay, 5715 yr, calculate the age of the shroud.
Ch.21 - Nuclear Chemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 21, Problem 43
Potassium-40 decays to argon-40 with a half-life of 1.27 * 109 yr. What is the age of a rock in which the mass ratio of 40Ar to 40K is 4.2?
 Verified step by step guidance
Verified step by step guidance1
Identify the decay process: Potassium-40 (\(^{40}\text{K}\)) decays to Argon-40 (\(^{40}\text{Ar}\)) through a radioactive decay process.
Use the given half-life of \(^{40}\text{K}\), which is 1.27 \times 10^9\) years, to determine the decay constant (\(\lambda\)) using the formula \(\lambda = \frac{\ln(2)}{\text{half-life}}\).
Apply the formula for radioactive decay: \(N_t = N_0 e^{-\lambda t}\), where \(N_t\) is the number of \(^{40}\text{K}\) atoms remaining, \(N_0\) is the initial number of \(^{40}\text{K}\) atoms, and \(t\) is the time elapsed.
Relate the mass ratio of \(^{40}\text{Ar}\) to \(^{40}\text{K}\) to the number of atoms: \(\frac{N_{\text{Ar}}}{N_{\text{K}}} = 4.2\), where \(N_{\text{Ar}}\) is the number of \(^{40}\text{Ar}\) atoms and \(N_{\text{K}}\) is the number of \(^{40}\text{K}\) atoms.
Solve for the age of the rock (\(t\)) using the relationship between \(N_{\text{Ar}}\), \(N_{\text{K}}\), and the decay constant \(\lambda\).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Radioactive Decay
Radioactive decay is the process by which an unstable atomic nucleus loses energy by emitting radiation. This decay occurs at a predictable rate characterized by the half-life, which is the time required for half of the radioactive substance to transform into a different element or isotope.
Recommended video:
Guided course

Rate of Radioactive Decay
Half-Life
Half-life is a specific time period in which half of a given quantity of a radioactive isotope decays into its daughter product. For potassium-40, the half-life is 1.27 billion years, meaning that after this time, half of the original potassium-40 will have decayed into argon-40.
Recommended video:
Guided course

Zero-Order Half-life
Mass Ratio and Age Calculation
The mass ratio of daughter to parent isotopes can be used to determine the age of a rock through the equation derived from the decay law. By knowing the mass ratio of argon-40 to potassium-40 and the half-life, one can calculate the time elapsed since the rock formed, providing an estimate of its age.
Recommended video:
Guided course
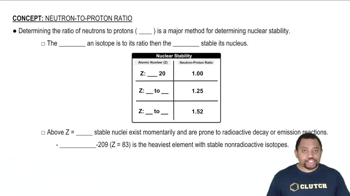
Neutron-Proton Ratio
Related Practice
Textbook Question
Textbook Question
The thermite reaction, Fe2O31s2 + 2 Al1s2 ¡2 Fe1s2 + Al2O31s2, H = -851.5 kJ>mol, is one of the most exothermic reactions known. Because the heat released is sufficient to melt the iron product, the reaction is used to weld metal under the ocean. How much heat is released per mole of Al2O3 produced? How does this amount of thermal energy compare with the energy released when 2 mol of protons and 2 mol of neutrons combine to form 1 mol of alpha particles?
