Textbook Question
Give the symbol for (b) an alpha particle.

 Verified step by step guidance
Verified step by step guidance
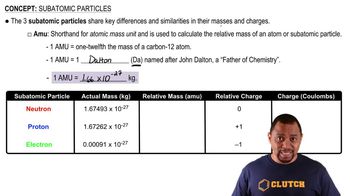
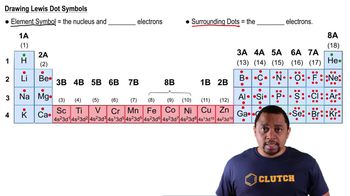
Give the symbol for (b) an alpha particle.
Give the symbol for (c) gamma radiation.
Give the symbol for (b) a beta particle.
Give the symbol for (c) a positron.
Write balanced nuclear equations for the following processes: (a) rubidium-90 undergoes beta emission.