Textbook Question
Indicate the number of protons and neutrons in the following nuclei: (b) 193Tl.
10
views
1
rank

 Verified step by step guidance
Verified step by step guidance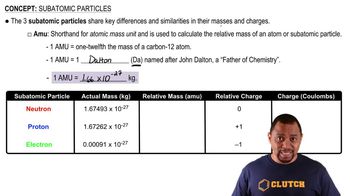
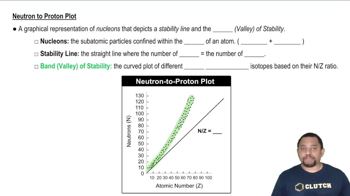
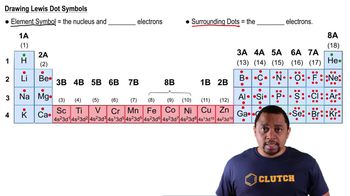
Indicate the number of protons and neutrons in the following nuclei: (b) 193Tl.
Indicate the number of protons and neutrons in the following nuclei: (c) neptunium-237.
Give the symbol for (b) an alpha particle.
Give the symbol for (c) gamma radiation.