Indicate whether each of the following nuclides lies within the belt of stability in Figure 21.2: (a) neon-24. For any that do not, describe a nuclear decay process that would alter the neutron-to-proton ratio in the direction of increased stability. [Section 21.2]

Complete and balance the following nuclear equations by supplying the missing particle: (b) 40₁₉K + 0₋₁e → ? (c) ? + 4₂He → 30₁₄Si + 1₁H
 Verified step by step guidance
Verified step by step guidanceKey Concepts
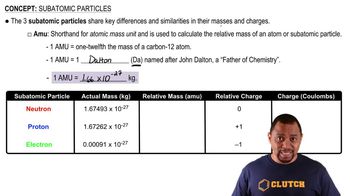
Nuclear Reactions

Balancing Nuclear Equations

Types of Particles in Nuclear Reactions
Write the balanced nuclear equation for the reaction represented by the diagram shown here. [Section 21.2]
In the sketch below, the red spheres represent protons and the gray spheres represent neutrons. (c) Based on its atomic number and mass number, do you think the product nucleus is stable or radioactive? [Section 21.3]
The steps below show three of the steps in the radioactive decay chain for 23290Th. The half-life of each isotope is shown below the symbol of the isotope. (a) Identify the type of radioactive decay for each of the steps (i), (ii), and (iii). [Sections 21.2 and 21.4]
The steps below show three of the steps in the radioactive decay chain for 23290Th. The half-life of each isotope is shown below the symbol of the isotope. (d) The next step in the decay chain is an alpha emission. What is the next isotope in the chain? [Sections 21.2 and 21.4]
