Textbook Question
The accompanying graph illustrates the decay of 8842Mo, which decays via positron emission. (d) What is the product of the decay process? [Section 21.4]
1
views

 Verified step by step guidance
Verified step by step guidance
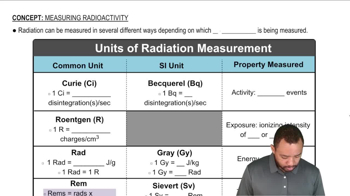

The accompanying graph illustrates the decay of 8842Mo, which decays via positron emission. (d) What is the product of the decay process? [Section 21.4]
All the stable isotopes of boron, carbon, nitrogen, oxygen, and fluorine are shown in the accompanying chart (in red), along with their radioactive isotopes with t1>2 7 1 min (in blue). (b) Which radioactive isotopes are most likely to decay by beta emission? [Sections 21.2, 21.4, and 21.5]
Indicate the number of protons and neutrons in the following nuclei: (b) 193Tl.
Indicate the number of protons and neutrons in the following nuclei: (c) neptunium-237.