The accompanying graph illustrates the decay of 8842Mo, which decays via positron emission. (b) What is the rate constant for the decay? [Section 21.4]
Ch.21 - Nuclear Chemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 21, Problem 7
A 65-kg person is accidentally exposed for 240 s to a 15-mCi source of beta radiation coming from a sample of 90Sr. (a) What is the activity of the radiation source in disintegrations per second? In becquerels? (b) Each beta particle has an energy of 8.75 * 10^-14 J, and 7.5% of the radiation is absorbed by the person. Assuming that the absorbed radiation is spread over the person’s entire body, calculate the absorbed dose in rads and in grays. (c) If the RBE of the beta particles is 1.0, what is the effective dose in mrem and in sieverts? (d) Is the radiation dose equal to, greater than, or less than that for a typical mammogram (300 mrem)?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Convert the activity of the radiation source from millicuries (mCi) to disintegrations per second. Use the conversion factor: 1 Ci = 3.7 × 10^10 disintegrations per second. Therefore, 1 mCi = 3.7 × 10^7 disintegrations per second.
Step 2: Convert the activity from disintegrations per second to becquerels (Bq). Note that 1 Bq is equivalent to 1 disintegration per second.
Step 3: Calculate the absorbed dose in rads. First, determine the total energy absorbed by the person using the formula: Energy absorbed = (Activity in Bq) × (Energy per beta particle) × (Exposure time in seconds) × (Fraction absorbed). Then, convert the energy absorbed to rads using the conversion factor: 1 rad = 0.01 J/kg.
Step 4: Convert the absorbed dose from rads to grays. Use the conversion factor: 1 gray = 100 rads.
Step 5: Calculate the effective dose in mrem and sieverts. Use the formula: Effective dose = Absorbed dose × RBE. Convert the effective dose from rads to mrem using the conversion factor: 1 rad = 100 mrem, and from grays to sieverts using the conversion factor: 1 gray = 1 sievert. Compare the effective dose to the typical mammogram dose of 300 mrem.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Radioactivity and Disintegration
Radioactivity is the process by which unstable atomic nuclei lose energy by emitting radiation. The activity of a radioactive source is measured in disintegrations per second, indicating how many nuclei decay in a given time. The SI unit for activity is the becquerel (Bq), where 1 Bq equals 1 disintegration per second. Understanding this concept is crucial for calculating the activity of the radiation source in the given problem.
Recommended video:
Guided course
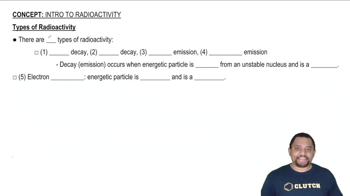
Types of Radioactivity
Absorbed Dose and Radiation Units
The absorbed dose quantifies the amount of energy deposited by ionizing radiation in a material, typically measured in rads or grays. One rad is equivalent to 0.01 joules of energy absorbed per kilogram of tissue, while one gray equals one joule per kilogram. In this context, calculating the absorbed dose involves considering the energy of the beta particles and the percentage absorbed by the person, which is essential for determining the potential biological effects of the radiation exposure.
Recommended video:
Guided course
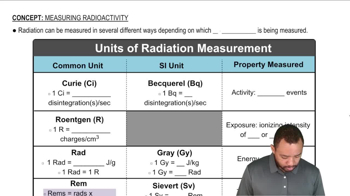
Units of Radiation Measurement
Relative Biological Effectiveness (RBE) and Effective Dose
Relative Biological Effectiveness (RBE) is a factor used to compare the biological effects of different types of radiation. It accounts for the varying damage caused by different radiation types, with beta particles typically having an RBE of 1.0. The effective dose, measured in sieverts (Sv) or millirem (mrem), incorporates the RBE to assess the overall risk of radiation exposure to human health. This concept is vital for evaluating the potential harm from the radiation dose received by the person.
Recommended video:
Guided course

Photoelectric Effect
Related Practice
Textbook Question
Textbook Question
The accompanying graph illustrates the decay of 8842Mo, which decays via positron emission. (c) What fraction of the original sample of 8842Mo remains after 12 min? [Section 21.4]
1
views
Textbook Question
The accompanying graph illustrates the decay of 8842Mo, which decays via positron emission. (d) What is the product of the decay process? [Section 21.4]
1
views
Textbook Question
All the stable isotopes of boron, carbon, nitrogen, oxygen, and fluorine are shown in the accompanying chart (in red), along with their radioactive isotopes with t1>2 7 1 min (in blue). (b) Which radioactive isotopes are most likely to decay by beta emission? [Sections 21.2, 21.4, and 21.5]
2
views
Textbook Question
Indicate the number of protons and neutrons in the following nuclei: (b) 193Tl.
10
views
1
rank
