Using standard reduction potentials (Appendix E), calculate the standard emf for each of the following reactions: (a) Cl21g2 + 2 I-1aq2 ¡ 2 Cl-1aq2 + I21s2

Using standard reduction potentials (Appendix E), calculate the standard emf for each of the following reactions: (d) 2 NO3-1aq2 + 8 H+1aq2 + 3 Cu1s2 ¡ 2 NO1g2 + 4 H2O1l2 + 3 Cu2+1aq2
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Standard Reduction Potentials

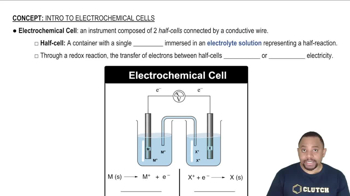
Electrochemical Cell and EMF
Balancing Redox Reactions

Using standard reduction potentials (Appendix E), calculate the standard emf for each of the following reactions: (b) Ni1s2 + 2 Ce4+1aq2 ¡ Ni2+1aq2 + 2 Ce3+1aq2
Using standard reduction potentials (Appendix E), calculate the standard emf for each of the following reactions: (c) Fe1s2 + 2 Fe3+1aq2 ¡ 3 Fe2+1aq2
The standard reduction potentials of the following halfreactions are given in Appendix E:
Ag+(aq) + e- → Ag(s)
Cu2+(aq) + 2 e- → Cu(s)
Ni2+(aq) + 2 e- → Ni(s)
Cr3+(aq) + 3 e- → Cr(s)
(a) Determine which combination of these half-cell reactions leads to the cell reaction with the largest positive cell potential and calculate the value.
(b) Determine which combination of these half-cell reactions leads to the cell reaction with the smallest positive cell potential and calculate the value.
